Lachnospiraceae-bacterium alleviates ischemia-reperfusion injury in steatotic donor liver by inhibiting ferroptosis via the Foxo3-Alox15 signaling pathway
- PMID: 39882747
- PMCID: PMC11784649
- DOI: 10.1080/19490976.2025.2460543
Lachnospiraceae-bacterium alleviates ischemia-reperfusion injury in steatotic donor liver by inhibiting ferroptosis via the Foxo3-Alox15 signaling pathway
Abstract
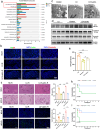
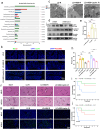
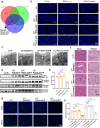
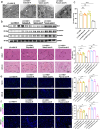
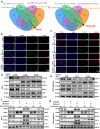
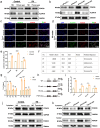
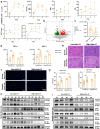
Ischemia-reperfusion injury (IRI) is a major obstacle in liver transplantation, especially with steatotic donor livers. Dysbiosis of the gut microbiota has been implicated in modulating IRI, and Lachnospiraceae plays a pivotal role in regulating host inflammatory and immune responses, but its specific role in liver transplantation IRI remains unclear. This study explores whether Lachnospiraceae can mitigate IRI and its underlying mechanisms. We found Lachnospiraceae-bacterium (Lachn.) abundance was significantly reduced in rats with liver cirrhosis. Lachn.-treated rats exhibited improved intestinal permeability, reduced IRI severity in both normal and steatotic donor livers, and decreased levels of neutrophil and macrophage infiltration, and inflammatory cytokines. Multi-omics analysis revealed elevated pyruvate levels in transplanted livers after Lachn. treatment, alongside reduced Alox15 and Foxo3 expression. Mechanistically, Lachn.-derived pyruvate inhibited Alox15 expression and reduced ferroptosis in normal and steatotic donor livers. Furthermore, reduced nuclear translocation of Foxo3 further suppressed Alox15 expression, alleviating IRI, especially in steatotic donor livers. Clinical samples confirmed reduced donor livers IRI in cirrhotic recipients with high Lachn. abundance after liver transplantation. In conclusion, Lachn. alleviates IRI in steatotic donor liver transplantation by inhibiting ferroptosis via the Foxo3-Alox15 axis, providing a potential therapeutic strategy to modulate gut microbiota to alleviate IRI following liver transplantation.
Keywords: Alox15; Lachnospiraceae-bacterium; ferroptosis; liver transplantation; steatotic donor livers.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
