A hierarchical pathway for assembly of the distal appendages that organize primary cilia
- PMID: 39882846
- PMCID: PMC11984956
- DOI: 10.7554/eLife.85999
A hierarchical pathway for assembly of the distal appendages that organize primary cilia
Abstract
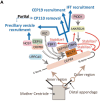
Distal appendages are ninefold symmetric blade-like structures attached to the distal end of the mother centriole. These structures are critical for the formation of the primary cilium, by regulating at least four critical steps: preciliary vesicle recruitment, recruitment and initiation of intraflagellar transport (IFT), and removal of CP110. While specific proteins that localize to the distal appendages have been identified, how exactly each protein functions to achieve the multiple roles of the distal appendages is poorly understood. Here, we comprehensively analyze known and newly discovered distal appendage proteins (CEP83, SCLT1, CEP164, TTBK2, FBF1, CEP89, KIZ, ANKRD26, PIDD1, LRRC45, NCS1, CEP15) for their precise localization, order of recruitment, and their roles in each step of cilia formation. Using CRISPR-Cas9 knockouts, we show that the order of the recruitment of the distal appendage proteins is highly interconnected and a more complex hierarchy. Our analysis highlights two protein modules, CEP83-SCLT1 and CEP164-TTBK2, as critical for structural assembly of distal appendages. Functional assays revealed that CEP89 selectively functions in the RAB34+ vesicle recruitment, while deletion of the integral components, CEP83-SCLT1-CEP164-TTBK2, severely compromised all four steps of cilium formation. Collectively, our analyses provide a more comprehensive view of the organization and the function of the distal appendage, paving the way for molecular understanding of ciliary assembly.
Keywords: cell biology; centrosome; cilia; ciliogenesis; distal appendage; human; super-resolution microscopy.
© 2025, Kanie et al.
Conflict of interest statement
TK, BL, JL, SF, AG, PJ No competing interests declared
Figures





















Update of
-
A hierarchical pathway for assembly of the distal appendages that organize primary cilia.bioRxiv [Preprint]. 2023 Jan 10:2023.01.06.522944. doi: 10.1101/2023.01.06.522944. bioRxiv. 2023. Update in: Elife. 2025 Jan 30;14:e85999. doi: 10.7554/eLife.85999. PMID: 36711481 Free PMC article. Updated. Preprint.
References
-
- Bayas C, Diezmann AV, Gustavsson AK, Moerner WE. Easy-DHPSF 2.0: open-source software for three-dimensional localization and two-color registration of single molecules with nanoscale accuracy. Protocol Exchange. 2019;01:e9151/v1. doi: 10.21203/rs.2.9151/v1. - DOI
MeSH terms
Grants and funding
- P20 GM103639/GM/NIGMS NIH HHS/United States
- R35 GM155365/GM/NIGMS NIH HHS/United States
- P20GM103447/GM/NIGMS NIH HHS/United States
- P30 CA225520/CA/NCI NIH HHS/United States
- R01 GM114276/GM/NIGMS NIH HHS/United States
- R01GM114276/GM/NIGMS NIH HHS/United States
- R35 GM151013/GM/NIGMS NIH HHS/United States
- R01GM121565/GM/NIGMS NIH HHS/United States
- S10 OD028536/OD/NIH HHS/United States
- R35GM155365/GM/NIGMS NIH HHS/United States
- C-2064-20210327/Welch Foundation
- RR200025/Cancer Prevention and Research Institute of Texas
- 1R35GM151013/GM/NIGMS NIH HHS/United States
- R00GM134187/GM/NIGMS NIH HHS/United States
- P20 GM103447/GM/NIGMS NIH HHS/United States
- R00 GM134187/GM/NIGMS NIH HHS/United States
- R01 GM121565/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

