Correlation between parathyroid adenoma volume and perioperative outcomes in primary hyperparathyroidism: Does the size matter?
- PMID: 39883321
- PMCID: PMC11961466
- DOI: 10.1007/s13304-025-02086-4
Correlation between parathyroid adenoma volume and perioperative outcomes in primary hyperparathyroidism: Does the size matter?
Abstract
Background: Primary hyperparathyroidism (PHPT) due to a parathyroid adenoma stands as one of the most prevalent endocrinological disorders, with focused parathyroidectomy being the established therapeutic strategy.
Aim: This study aims to investigate whether the volume of the pathological gland influences perioperative outcomes and postoperative morbidity.
Methods: A retrospective analysis was conducted on data from 141 patients who underwent focused parathyroidectomy for PHPT at the University Hospital of Basel between 2007 and 2022.
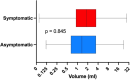
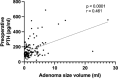
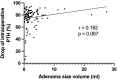
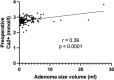
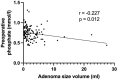
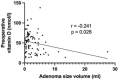
Results: A total of 141 patients underwent surgery, with a mean age of 57.2 years and prevalence of women (64.5%).The volume of the lesion was divided into three groups (low < 1 ml, middle 1-1.99 ml, large > 2 ml) based on pathological specimen analysis. Preoperative calcium and parathyroid hormone (PTH) values were significantly higher in the large volume group compared to the low volume group (p < 0.05), while phosphate and vitamin D values were significantly lower (p < 0.05). A comparison of adenoma volume in symptomatic patients with asymptomatic patients revealed no statistically significant difference (p = 0.845) and the volume of the gland of any group did not influence the length of the operation (p = 0.173) and the perioperative morbidity (p = 0.108).
Conclusion: Compared to a volume of less than 1 ml, a parathyroid gland volume greater than 2 ml was associated with higher preoperative PTH and calcium levels and lower phosphate and vitamin D levels. The volume of the parathyroid gland does not seem to impact the clinical manifestations, or the incidence of perioperative complications.
Keywords: Parathyroid adenoma; Parathyroidectomy; Postoperative complications; Primary hyperparathyroidism.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no conflict of interest. Research involving human participants and/or animals: The ethics committee and review board in our institute approved the study and treatment protocol. Informed consent: Informed consent was obtained from all patients who agreed to participate in the study.
Figures
References
-
- Duan K, Gomez Hernandez K, Mete O (2015) Clinicopathological correlates of hyperparathyroidism. J Clin Pathol 68(10):771–787. 10.1136/jclinpath-2015-203186 - PubMed
-
- Bilezikian JP, Khan AA, Silverberg SJ et al (2022) Evaluation and management of primary hyperparathyroidism: summary statement and guidelines from the fifth international workshop. J Bone Miner Res 37(11):2293–2314. 10.1002/jbmr.4677 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

