Psychometric properties and post-hoc CAT analysis of the pediatric PROMIS® item banks anxiety and depressive symptoms in a combined Swedish Child and Adolescent Psychiatry and School sample
- PMID: 39883384
- PMCID: PMC12064460
- DOI: 10.1007/s11136-025-03898-y
Psychometric properties and post-hoc CAT analysis of the pediatric PROMIS® item banks anxiety and depressive symptoms in a combined Swedish Child and Adolescent Psychiatry and School sample
Abstract
Purpose: The objective of this study is to assess the psychometric properties and reliability of the Swedish Patient-Reported Outcomes Measurement Information System (PROMIS) item banks for anxiety and depressive symptoms with item response theory analysis and post-hoc computerized adaptive testing in a combined Swedish Child and Adolescent Psychiatry (CAP) and school sample.
Methods: Participants (n = 928, age 12-20) were recruited from junior and high schools and Child and Adolescent Psychiatry Clinics in the region of Västerbotten. Unidimensionality, local independence, and monotonicity was tested. We fitted a graded response model to the data and tested differential item functioning (DIF) for sex, age group, sample type, and language (Swedish vs. U.S.). Moreover, a post-hoc computer adaptive testing (CAT) simulation was performed. All analysis were made in R.
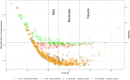
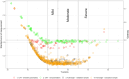
Results: Unidimensionality, local independence, and monotonicity were acceptable. The graded response model yielded acceptable item fit, discriminative, and threshold values for all items in both item banks. DIF for language (Swedish vs. U.S.) was found for two items from the anxiety and one item from the depressive symptoms item banks. A Stocking-lord transformation was used for the items displaying language DIF, and post-hoc CAT simulations were performed. The post-hoc CAT simulation showed reliability around 0.9 for both Swedish and official U.S. item parameters T-scores calibration from within normal limits to severe anxiety and depressive symptoms.
Conclusion: The Swedish pediatric PROMIS item banks of anxiety and depressive symptoms are appropriate to assess mild to severe symptoms of anxiety and depressive symptoms in Swedish school- and CAP samples.
Keywords: Child- and adolescent psychiatry; Computer adaptive testing; Depressive symptoms; Differential item functioning; Graded response model; Item response theory.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: Dr. J. Chaplin is on the Board of Directors, the PROMIS Health Organization. Dr. I. Dennhag is a member of the PROMIS Health Organization. None of the authors have any financial or non-financial conflicts of interest. Consent to participate: Informed oral and written consent was obtained from all respondents and additional parental consent for respondents 15 years or younger. Consent to publish: Not applicable. Ethical approval: This study was approved by the Swedish Regional Ethical Review Board in Sweden (Number 2018/59-31).
Figures
References
-
- Cella, D., Riley, W., Stone, A., Rothrock, N., Reeve, B., Yount, S., Amtmann, D., Bode, R., Buysse, D., Choi, S., Cook, K., Devellis, R., Dewalt, D., Fries, J. F., Gershon, R., Hahn, E. A., Lai, J.-S., Pilkonis, P., Revicki, D., … Hays, R. (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology,63(11), 1179–1194. 10.1016/j.jclinepi.2010.04.011 - DOI - PMC - PubMed
-
- Cella, F. D., Yount, F. S., Rothrock, F. N., Gershon, F. R., Cook, F. K., Reeve, F. B., Ader, F. D., Fries, F. J., Bruce, F. B., & Rose, F. M. (2007). The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Medical Care,45(5 Suppl 1), S3–S11. 10.1097/01.mlr.0000258615.42478.55 - DOI - PMC - PubMed
-
- Irwin, D., Stucky, B., Langer, M., Thissen, D., DeWitt, E., Lai, J.-S., Varni, J., Yeatts, K., & DeWalt, D. (2010). An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation—Official Journal of the International Society of Quality of Life Research,19(4), 595–607. 10.1007/s11136-010-9619-3 - DOI - PMC - PubMed
-
- Irwin, D., Stucky, B., Thissen, D., DeWitt, E., Lai, J., Yeatts, K., Varni, J., & DeWalt, D. (2010). Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation—Official Journal of the International Society of Quality of Life Research,19(4), 585–594. 10.1007/s11136-010-9618-4 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

