A Phase 3B, Open-Label Study to Evaluate the Immunogenicity and Safety of the Quadrivalent Meningococcal Nimenrix® Vaccine When Given to Healthy Infants at 3 and 12 Months of Age
- PMID: 39883399
- PMCID: PMC11829884
- DOI: 10.1007/s40121-024-01098-8
A Phase 3B, Open-Label Study to Evaluate the Immunogenicity and Safety of the Quadrivalent Meningococcal Nimenrix® Vaccine When Given to Healthy Infants at 3 and 12 Months of Age
Abstract
Introduction: Infants and young children typically have the highest age-related risk of invasive meningococcal disease. The immunogenicity and safety of a single primary dose and a booster of a meningococcal A/C/W/Y tetanus toxoid conjugate vaccine (MenACWY-TT; Nimenrix®) in infants were evaluated.
Methods: In this phase 3b, open-label, single-arm study, healthy 3-month-old infants received a single Nimenrix dose followed by a booster at age 12 months (1 + 1 series). Functional antibodies before and 1 month after each vaccination were evaluated with serum bactericidal antibody assays using rabbit (rSBA) or human (hSBA) complement for each A/C/W/Y serogroup. Primary endpoints were rSBA seroprotection (titers ≥ 1:8) rates and geometric mean titers (GMTs); supportive secondary endpoints included hSBA seroprotection (titers ≥ 1:4) rates and GMTs. Local reactions and systemic events occurring within 7 days, adverse events (AEs), serious AEs, and newly diagnosed chronic medical conditions following vaccination were assessed.
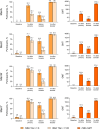
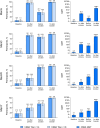
Results: Overall, 147 and 143 participants received the primary and booster Nimenrix doses, respectively. rSBA seroprotection rates across serogroups were 82.3-91.1% at 1 month after the primary dose and increased to 100% at 1 month after the booster. rSBA GMTs were considerably higher after the booster (1299.5‒2714.1) than after the primary dose (54.7‒202.4). In hSBA evaluations performed as supportive to rSBA evaluations, seroprotection rates increased from 38.8 to 95.5% after the primary dose to 100% after the booster, with corresponding GMT increases (8.8‒149.8 to 1208.4‒7299.6). Local reactions and most systemic events were mild to moderate in severity; no new safety concerns were identified.
Conclusion: Nimenrix given at ages 3 and 12 months had a favorable safety profile and elicited protective immune responses and robust anamnestic booster responses across A/C/W/Y serogroups. These results provide important support for this alternative Nimenrix 1 + 1 immunization schedule for infants < 6 months, allowing flexibility in infant meningococcal immunization.
Trial registration: ClinicalTrials.gov, NCT04819113.
Keywords: Immunogenicity; Infants; Invasive meningococcal disease; MenACWY-TT; Nimenrix; Safety; Vaccination schedules; rSBA.
Plain language summary
Infants and young children typically have the highest age-related risk of invasive meningococcal disease, a rare but potentially devastating illness. Nimenrix is a vaccine that protects against meningococcal types A, C, W, and Y, which are among the most common causes of invasive meningococcal disease. Currently, for infants 6 weeks to < 6 months of age, Nimenrix is recommended to be administered as 2 initial doses, and then a booster dose at age 12 months (2 + 1 schedule). In this study, healthy infants received Nimenrix at 3 months (primary dose) and 12 months (booster) of age to assess the possible use of an alternative 1 + 1 schedule. Blood samples from vaccinated infants were evaluated for the presence of antibodies able to kill bacteria for types A/C/W/Y at levels that are considered protective. Before vaccination, 0–8% of infants had antibodies at those levels across the A/C/W/Y types. Percentages increased to range from 82 to 91% at 1 month after the primary dose, then decreased before the booster but reached 100% against each A/C/W/Y type at 1 month after the booster. Antibody levels after the booster were considerably higher than those after the primary dose. The Nimenrix 1 + 1 schedule was well tolerated and safe, reactions were mostly mild to moderate in severity, and no safety concerns were identified. Overall, these results provide important support for the Nimenrix 1 + 1 immunization schedule for infants as young as 3 months, allowing flexibility in infant immunization against meningococcal disease.
© 2025. Pfizer Inc.
Conflict of interest statement
Declarations. Conflict of Interest: Susanna Koski and Federico Martinon-Torres acted as principal investigators for the study reported in this manuscript, which was sponsored by Pfizer. Susanna Koski and Mika Rämet are employees of Finnish Vaccine Research (FVR), which conducts clinical vaccine studies for many major vaccine manufacturers, including Pfizer. Federico Martinon-Torres has acted as principal investigator for other studies sponsored by Abbot, Cubist, GlaxoSmithKline, Janssen, Medimmune, MSD, Novavax, Novartis, Pfizer, Regeneron, Roche, Sanofi Pasteur, and Seqirus, with honoraria paid to his institution; and has consulting or advisory relationships with GlaxoSmithKline, Janssen, MSD, Pfizer, Sanofi Pasteur, and Seqirus. All other authors are current or former employees of Pfizer and may hold stock or stock options in the company. Ethical Approval: The study was conducted in accordance with consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences Ethical Guidelines, applicable International Conference on Harmonisation Good Clinical Practice guidelines, and applicable laws and regulations. The protocol and related documents were reviewed and approved by the Ethics Committee Hospital District of Southwest Finland (study sites in Finland; reference number 7/1800/2021), Komisja Bioetyczna przy Okregowej Izbie Lekarskiej w Krakowie (Poland; reference number 11/KBL/OIL/2021), and Comité de Ética de Investigación con Medicamentos del Hospital Universitario 12 de Octubre (Spain; reference number 21/040) before study initiation. A parent or legal guardian of each study participant provided written informed consent before the participant was enrolled.
Figures

Similar articles
-
Immunogenicity and Safety of a Quadrivalent Meningococcal Conjugate Vaccine Versus Nimenrix in Healthy Adolescents: A Randomized Phase IIIb Multicenter Study.Infect Dis Ther. 2024 Aug;13(8):1835-1859. doi: 10.1007/s40121-024-01009-x. Epub 2024 Jul 2. Infect Dis Ther. 2024. PMID: 38955966 Free PMC article.
-
Immunogenicity and safety of a quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) administered to adults aged 56 Years and older: results of an open-label, randomized, controlled trial.Drugs Aging. 2013 May;30(5):309-19. doi: 10.1007/s40266-013-0065-0. Drugs Aging. 2013. PMID: 23494214 Free PMC article. Clinical Trial.
-
Immunogenicity and Safety of a Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) Administered with Routine Pediatric Vaccines: A European Randomized Controlled Trial.Infect Dis Ther. 2025 Aug;14(8):1843-1865. doi: 10.1007/s40121-025-01190-7. Epub 2025 Jul 15. Infect Dis Ther. 2025. PMID: 40665158 Free PMC article.
-
Meningococcal quadrivalent (serogroups A, C, W135 and Y) tetanus toxoid conjugate vaccine (Nimenrix™).Drugs. 2012 Dec 24;72(18):2407-30. doi: 10.2165/11209580-000000000-00000. Drugs. 2012. PMID: 23231026 Review.
-
Review of clinical studies comparing meningococcal serogroup C immune responses induced by MenACWY-TT and monovalent serogroup C vaccines.Hum Vaccin Immunother. 2021 Jul 3;17(7):2205-2215. doi: 10.1080/21645515.2020.1855952. Epub 2021 Feb 19. Hum Vaccin Immunother. 2021. PMID: 33606596 Free PMC article. Review.
References
-
- Thompson MJ, Ninis N, Perera R, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367:397–403. - PubMed
-
- Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37:2768–82. - PubMed
-
- Stein-Zamir C, Shoob H, Sokolov I, et al. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr Infect Dis J. 2014;33:777–9. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

