Monocytes and interstitial macrophages contribute to hypoxic pulmonary hypertension
- PMID: 39883518
- PMCID: PMC11910231
- DOI: 10.1172/JCI176865
Monocytes and interstitial macrophages contribute to hypoxic pulmonary hypertension
Abstract
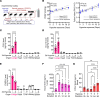
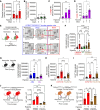
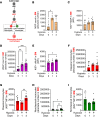
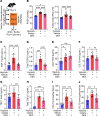
Hypoxia is a major cause of pulmonary hypertension (PH) worldwide, and it is likely that interstitial pulmonary macrophages contribute to this vascular pathology. We observed in hypoxia-exposed mice an increase in resident interstitial macrophages, which expanded through proliferation and expressed the monocyte recruitment ligand CCL2. We also observed an increase in CCR2+ macrophages through recruitment, which express the protein thrombospondin-1, which functionally activates TGF-β to cause vascular disease. Blockade of monocyte recruitment with either CCL2-neutralizing antibody treatment or CCR2 deficiency in the bone marrow compartment suppressed hypoxic PH. These data were supported by analysis of plasma samples from humans who traveled from low (225 m) to high (3500 m) elevation, revealing an increase in thrombospondin-1 and TGF-β expression following ascent, which was blocked by dexamethasone prophylaxis. In the hypoxic mouse model, dexamethasone prophylaxis recapitulated these findings by mechanistically suppressing CCL2 expression and CCR2+ monocyte recruitment. These data suggest a pathologic cross talk between 2 discrete interstitial macrophage populations, which can be therapeutically targeted.
Keywords: Chemokines; Hypoxia; Inflammation; Monocytes; Vascular biology.
Conflict of interest statement
Figures




Comment in
- Macrophages: Key conductors behind perivascular inflammation and vascular remodeling in hypoxia induced pulmonary hypertension doi: 10.1172/JCI190957
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

