Characterization of cognitive decline in long-duration type 1 diabetes by cognitive, neuroimaging, and pathological examinations
- PMID: 39883521
- PMCID: PMC11949075
- DOI: 10.1172/jci.insight.180226
Characterization of cognitive decline in long-duration type 1 diabetes by cognitive, neuroimaging, and pathological examinations
Abstract
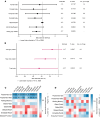
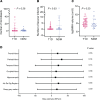
BACKGROUNDWe aimed to characterize factors associated with the under-studied complication of cognitive decline in aging people with long-duration type 1 diabetes (T1D).METHODSJoslin "Medalists" (n = 222; T1D ≥ 50 years) underwent cognitive testing. Medalists (n = 52) and age-matched nondiabetic controls (n = 20) underwent neuro- and retinal imaging. Brain pathology (n = 26) was examined. Relationships among clinical, cognitive, and neuroimaging parameters were evaluated.RESULTSCompared with controls, Medalists had worse psychomotor function and recall, which associated with female sex, lower visual acuity, reduced physical activity, longer diabetes duration, and higher inflammatory cytokines. On neuroimaging, compared with controls, Medalists had significantly lower total and regional brain volumes, equivalent to 9 years of accelerated aging, but small vessel disease markers did not differ. Reduced brain volumes associated with female sex, reduced psychomotor function, worse visual acuity, longer diabetes duration, and higher inflammation, but not with glycemic control. Worse cognitive function, lower brain volumes, and diabetic retinopathy correlated with thinning of the outer retinal nuclear layer. Worse baseline visual acuity associated with declining psychomotor function in longitudinal analysis. Brain volume mediated the association between visual acuity and psychomotor function by 57%. Brain pathologies showed decreased volumes, but predominantly mild vascular or Alzheimer's-related pathology.CONCLUSION To our knowledge, this is the first comprehensive study of cognitive function, neuroimaging, and pathology in aging T1D individuals demonstrated that cognitive decline was related to parenchymal rather than neurovascular abnormalities, unlike type 2 diabetes, suggestive of accelerated aging in T1D. Improving visual acuity could perhaps be an important preventive measure against cognitive decline in people with T1D.FUNDINGThe Beatson Foundation, NIH/NIDDK grants 3P30DK036836-34S1 and P30DK036836-37, and Mary Iacocca fellowships.
Keywords: Aging; Dementia; Diabetes; Endocrinology.
Conflict of interest statement
Figures




References
-
- CDC. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/php/data-research/index.html Accessed January 27, 2024.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

