Multimodal integration of blood RNA and ctDNA reflects response to immunotherapy in metastatic urothelial cancer
- PMID: 39883530
- PMCID: PMC11949011
- DOI: 10.1172/jci.insight.186062
Multimodal integration of blood RNA and ctDNA reflects response to immunotherapy in metastatic urothelial cancer
Abstract
Background: Previously, we demonstrated that changes in circulating tumor DNA (ctDNA) are promising biomarkers for early response prediction (ERP) to immune checkpoint inhibitors (ICIs) in metastatic urothelial cancer (mUC). In this study, we investigated the value of whole-blood immunotranscriptomics for ERP-ICI and integrated both biomarkers into a multimodal model to boost accuracy.
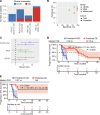
Methods: Blood samples of 93 patients were collected at baseline and after 2-6 weeks of ICI for ctDNA (n = 88) and immunotranscriptome (n = 79) analyses. ctDNA changes were dichotomized into increase or no increase, the latter including patients with undetectable ctDNA. For RNA model development, the cohort was split into discovery (n = 29), test (n = 29), and validation sets (n = 21). Finally, RNA- and ctDNA-based predictions were integrated in a multimodal model. Clinical benefit (CB) was defined as progression-free survival beyond 6 months.
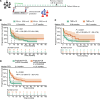
Results: Sensitivity (SN) and specificity (SP) of ctDNA increase for predicting non-CB (N-CB) was 59% and 92%, respectively. Immunotranscriptome analysis revealed upregulation of T cell activation, proliferation, and interferon signaling during treatment in the CB group, in contrast with N-CB patients. Based on these differences, a 10-gene RNA model was generated, reaching an SN and SP of 73% and 79%, respectively, in the test and 67% and 67% in the validation set for predicting N-CB. Multimodal model integration led to superior performance, with an SN and SP of 79% and 100%, respectively, in the validation cohort.
Conclusion: The combination of whole-blood immunotranscriptome and ctDNA in a multimodal model showed promise for ERP-ICI in mUC and accurately identified patients with N-CB.
Funding: Eurostars grant E! 114908 - PRECISE, Paul Speth Foundation (Bullseye project).
Keywords: Bioinformatics; Cancer immunotherapy; Immunology; Oncology; Urology.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

