A new computational framework for simulating airway resistance, fraction of exhaled nitric oxide, and diffusing capacity for nitric oxide
- PMID: 39883668
- PMCID: PMC11781630
- DOI: 10.1371/journal.pone.0311667
A new computational framework for simulating airway resistance, fraction of exhaled nitric oxide, and diffusing capacity for nitric oxide
Abstract
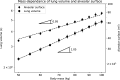
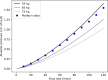
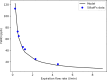
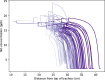
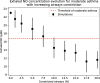
In this paper, we present a new computational framework for the simulation of airway resistance, the fraction of exhaled nitric oxide, and the diffusion capacity for nitric oxide in healthy and unhealthy lungs. Our approach is firstly based on a realistic representation of the geometry of healthy lungs as a function of body mass, which compares well with data from the literature, particularly in terms of lung volume and alveolar surface area. The original way in which this geometry is created, including an individual definition of the airways in the first seven generations of the lungs, makes it possible to consider the heterogeneous nature of the lungs in terms of perfusion and ventilation. In addition, a geometry can be easily modified to simulate various abnormalities, local or global (constriction, inflammation, perfusion defect). The natural variability of the lungs at constant body mass is also considered. The computational framework includes the possibility to simulate, on a given (possibly modified) geometry, a test to measure the flow resistance of the lungs (including its component due to the not fully developed flow in the first generations of lungs), a test to measure the concentration of nitric oxide in the exhaled air, and a test to measure the diffusion capacity for nitric oxide. This is implemented in the framework by solving different transport equations (momentum and convection/diffusion) describing these tests. Through numerous simulations, we demonstrate the ability of our model to reproduce results from the literature, both for healthy lungs and lungs of patients with asthma or chronic obstructive pulmonary disease. Such a computational framework, through the possibilities of numerous and rapid tests that it allows, sheds new light on experimental data by providing information on the phenomena that take place in the distal generations of the lungs, which are difficult to access with imaging.
Copyright: © 2025 Haut et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Weibel E. R., Morphometry of the human lung, Academic Press, 1963.
-
- West J. B., Respiratory physiology: The essentials (9th edition), Philadephia: Lippincott Williams and Wilkins, 2011.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

