ASGCL: Adaptive Sparse Mapping-based graph contrastive learning network for cancer drug response prediction
- PMID: 39883719
- PMCID: PMC11781687
- DOI: 10.1371/journal.pcbi.1012748
ASGCL: Adaptive Sparse Mapping-based graph contrastive learning network for cancer drug response prediction
Abstract
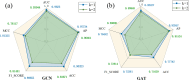
Personalized cancer drug treatment is emerging as a frontier issue in modern medical research. Considering the genomic differences among cancer patients, determining the most effective drug treatment plan is a complex and crucial task. In response to these challenges, this study introduces the Adaptive Sparse Graph Contrastive Learning Network (ASGCL), an innovative approach to unraveling latent interactions in the complex context of cancer cell lines and drugs. The core of ASGCL is the GraphMorpher module, an innovative component that enhances the input graph structure via strategic node attribute masking and topological pruning. By contrasting the augmented graph with the original input, the model delineates distinct positive and negative sample sets at both node and graph levels. This dual-level contrastive approach significantly amplifies the model's discriminatory prowess in identifying nuanced drug responses. Leveraging a synergistic combination of supervised and contrastive loss, ASGCL accomplishes end-to-end learning of feature representations, substantially outperforming existing methodologies. Comprehensive ablation studies underscore the efficacy of each component, corroborating the model's robustness. Experimental evaluations further illuminate ASGCL's proficiency in predicting drug responses, offering a potent tool for guiding clinical decision-making in cancer therapy.
Copyright: © 2025 Dong et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures










References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

