Impact of vein-to-vein time in patients with R/R LBCL treated with axicabtagene ciloleucel
- PMID: 39883946
- PMCID: PMC12155625
- DOI: 10.1182/bloodadvances.2024013656
Impact of vein-to-vein time in patients with R/R LBCL treated with axicabtagene ciloleucel
Abstract
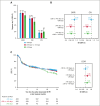
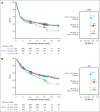
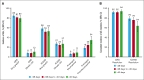
Chimeric antigen receptor (CAR) T-cell products axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel) are approved for relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Emerging evidence indicates that delayed CAR T-cell infusion, including prolonged time from leukapheresis to infusion, known as vein-to-vein time (V2Vt), may adversely impact clinical outcomes. We conducted a systematic literature review (SLR) and meta-analysis to identify differences in V2Vt in patients with R/R LBCL treated with axi-cel, tisa-cel, or liso-cel. The impact of V2Vt (<28 days vs ≥28 to <40 days vs ≥40 days) on effectiveness and safety outcomes was evaluated in patients treated with axi-cel enrolled in a post-authorization safety study using the Center for International Blood and Marrow Transplant Research data. SLR and meta-analysis showed that patients treated with axi-cel had the shortest median V2Vt (30.6 days) compared with tisa-cel (48.4 days) or liso-cel (35.9 days). Real-world analysis of patients treated with axi-cel demonstrated that V2Vt ≥40 days was associated with significantly lower complete response rate than V2Vt <28 days (odds ratio [OR], 0.61) or ≥28 to <40 days (OR, 0.66) and significantly worse overall survival than V2Vt <28 days (hazard ratio [HR], 1.33) or ≥28 to <40 days (HR, 1.36). Higher prolonged thrombocytopenia rates were observed in patients with axi-cel V2Vt ≥28 to <40 days or ≥40 days compared with <28 days (OR, 1.44 or 1.95, respectively). Together, these results show the impact of V2Vt on patient outcomes with axi-cel therapy and that earlier infusion with CD19-CAR therapies may be beneficial.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: F.L.L. has served in a consultancy or advisory role for Allogene, Amgen, Bluebird Bio, Bristol Myers Squibb/Celgene, Calibr, Caribou, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja; has received research funding from Kite/Gilead, Allogene, CERo Therapeutics, Novartis, Bluebird Bio, Bristol Myers Squibb, National Cancer Institute, and Leukemia & Lymphoma Society; has several patents held by the institution (unlicensed) in the field of cellular immunotherapy; has received travel, accommodations, or expenses from A2 Bio; and has served in an education or editorial role for Aptitude Health, American Society of Hematology, BioPharma Communications, CARE Education, Clinical Care Options Oncology, Imedex, and Society for Immunotherapy of Cancer. T.S. has served in a consultancy or advisory role for AstraZeneca, Pharmacyclics, Celgene, Juno, Kite/Gilead, and BeiGene; has been paid to participate in a speakers’ bureau by AstraZeneca, Bristol Myers Squibb, and BeiGene; and has received research funding from Bristol Myers Squibb, Pharmacyclics, Juno Therapeutics, Kite, AstraZeneca, BeiGene, Oncternal, TG Therapeutics, Ascentage Pharma, and Celgene. C.A.J. has been paid honoraria previously by Kite/Gilead, Novartis, Bristol Myers Squibb/Celgene, Bluebird Bio, Epizyme, Ipsen, Daiichi-Sankyo, Instill Bio, ImmPACT Bio, Caribou Bio, and Abintus Bio; has served in a consultancy or advisory role for Kite/Gilead, Novartis, Bristol Myers Squibb/Celgene, Bluebird Bio, Ipsen, Daiichi-Sankyo, Instill Bio, ImmPACT Bio, Caribou Bio, and Abintus Bio; and has received research funding from Kite/Gilead and Pfizer, and personal fees from Bristol Myers Squibb/Celgene. S.N. has served in a consultancy or advisory role for ad hoc advisory board for Iovance, Kite/Gilead, SmartImmune, and Legend; and has received travel, accommodations, and expenses from A2 Bio. S.A. has been paid honoraria previously by Kite, ADCT, Novartis, and Nektar; has served in a consultancy or advisory role for Myeloid, Nektar, and Tessa; and has received research funding from Xencor, Tessa, Nektar, Merck, and Bristol Myers Squibb. D.B.M. has been paid honoraria previously by Janssen; has served in a consultancy or advisory role for Janssen, Adaptive Biotechnologies, Kite, Bristol Myers Squibb, and Miltenyi Biotec; has received research funding from Kite, Adicet, Allogene, 2Seventy Bio, Fate Therapeutics, and Miltenyi Biotec; is a chronic graft-versus-host disease patent holder for ibrutinib as chronic graft-versus-host disease therapy, with no compensation; and has received personal fees from Janssen. Y.L. has served in a consultancy or advisory role for Kite/Gilead, Celgene/Bristol Myers Squibb, Juno/Bristol Myers Squibb, Bluebird Bio, Janssen, Legend BioTech, Gamida Cell, Novartis, Iovance, Takeda, Fosun Kite, and Pfizer; and has received research funding from Kite/Gilead, Celgene/Bristol Myers Squibb, Bluebird Bio, Janssen, Legend Biotech, Merck, Takeda, and Boston Scientific. M.A.L. has been paid honoraria and/or has served in a consultancy or advisory role for AbbVie, Acrotech, ADC Therapeutics, AstraZeneca, Astellas, Bristol Myers Squibb, Caribou, CRISPR, Daiichi Sankyo, EUSA, Fate Therapeutics, Genentech, GenMab, InstilBio, Ipsen, Janssen, Kite, Loxo, Miltenyi, MorphoSys, Novartis, Nurix, Pharmacyclics, Regeneron, Sanofi, Seagen, Takeda, and TG Therapeutics; and has received research funding from Bristol Myers Squibb, Curis, FATE Therapeutics, and Sana Therapeutics. B.T.H. has been paid an honorarium and has served in a consulting or advisory role for Kite; and has received research funding and travel, accommodations, and expenses from Kite. A.G. has been paid an honorarium from Kite; has served in a consulting or advisory role for Amgen, Atara, Bristol Myers Squibb, CRISPR Therapeutics, Kite, and Wugen Inc; and has received research funding from Amgen, Genentech, and Kite. Z-H.H. owns stock in Gilead and is an employee of Kite, a Gilead Company. M.T.H. has been paid an honorarium and has served in a consulting or advisory role for Pfizer; and is an employee of Kite, a Gilead Company. M.J.Z. reported being employed by RainCity Analytics. S.V. owns stock in Gilead; received travel, accommodation, and expenses from Kite; and is an employee of Kite, a Gilead Company. J.T. owns stock in Biomarin, Bristol Myers Squibb, Bayer, and Gilead; received travel, accommodation, and expenses from Kite; and is an employee of Kite, a Gilead Company. C.S. has received research support from Delta Hat Limited, and is an employee of Kite, a Gilead Company. H.S. owns stock in Gilead; and is an employee of Kite, a Gilead Company. C.F. has an immediate family member who has patents, royalties, or other intellectual property from Cellares; owns stock in Amgen; and is an employee of Kite, a Gilead Company. A.P. is an employee of Kite, a Gilead Company. H.M. is an employee of Kite, a Gilead Company. S.A.S. has been a paid speaker for Amgen, and is an employee of Kite, a Gilead Company. D.L.M. owns stock in Gilead, and is an employee of Kite, a Gilead Company. H.X. is an employee of Kite, a Gilead Company. M.C.P. has received fees for consulting or an advisory role from Bristol Myers Squibb; and has received research funding from Kite, Novartis, Bristol Myers Squibb, Janssen, and GlaxoSmithKline.
Figures




References
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
-
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

