Exploring the Ascorbate Requirement of the 2-Oxoglutarate-Dependent Dioxygenases
- PMID: 39883951
- PMCID: PMC11831678
- DOI: 10.1021/acs.jmedchem.4c02342
Exploring the Ascorbate Requirement of the 2-Oxoglutarate-Dependent Dioxygenases
Abstract
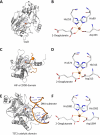
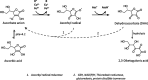
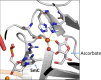
In humans, the 2-oxoglutarate-dependent dioxygenases (2-OGDDs) catalyze hydroxylation reactions involved in cell metabolism, the biosynthesis of small molecules, DNA and RNA demethylation, the hypoxic response and the formation of collagen. The reaction is catalyzed by a highly oxidizing ferryl-oxo species produced when the active site non-heme iron engages molecular oxygen. Enzyme activity is specifically stimulated by l-ascorbic acid (ascorbate, vitamin C), an effect not well mimicked by other reducing agents. In this perspective article we discuss the reliance of the 2-OGDDs on ascorbate availability. We draw upon findings from studies with different 2-OGDDs to piece together a comprehensive theory for the specific role of ascorbate in supporting enzyme activity. Our discussion centers on the capacity for ascorbate to act as an efficient radical scavenger and its propensity to reduce and chelate transition metals. In addition, we consider the evidence supporting stereospecific binding of ascorbate in the enzyme active site.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Synthesis of 5-hydroxyectoine from ectoine: crystal structure of the non-heme iron(II) and 2-oxoglutarate-dependent dioxygenase EctD.PLoS One. 2010 May 14;5(5):e10647. doi: 10.1371/journal.pone.0010647. PLoS One. 2010. PMID: 20498719 Free PMC article.
-
2-oxoglutarate-dependent dioxygenases: A renaissance in attention for ascorbic acid in plants.PLoS One. 2020 Dec 8;15(12):e0242833. doi: 10.1371/journal.pone.0242833. eCollection 2020. PLoS One. 2020. PMID: 33290424 Free PMC article.
-
Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate.Biochem Biophys Res Commun. 2013 Oct 4;439(4):522-7. doi: 10.1016/j.bbrc.2013.09.010. Epub 2013 Sep 8. Biochem Biophys Res Commun. 2013. PMID: 24021282 Free PMC article.
-
Ascorbic acid metabolism and functions: A comparison of plants and mammals.Free Radic Biol Med. 2018 Jul;122:116-129. doi: 10.1016/j.freeradbiomed.2018.03.033. Epub 2018 Mar 20. Free Radic Biol Med. 2018. PMID: 29567393 Free PMC article. Review.
-
Regulation of the 2-oxoglutarate-dependent dioxygenases and implications for cancer.Biochem Soc Trans. 2014 Aug;42(4):945-51. doi: 10.1042/BST20140118. Biochem Soc Trans. 2014. PMID: 25109984 Review.
Cited by
-
Biochemical investigations using mass spectrometry to monitor JMJD6-catalysed hydroxylation of multi-lysine containing bromodomain-derived substrates.RSC Chem Biol. 2025 Feb 24;6(4):642-656. doi: 10.1039/d4cb00311j. eCollection 2025 Apr 2. RSC Chem Biol. 2025. PMID: 40046450 Free PMC article.
-
Differential control of RNA demethylase activity and selectivity by cofactor ascorbate.bioRxiv [Preprint]. 2025 May 8:2025.05.06.652568. doi: 10.1101/2025.05.06.652568. bioRxiv. 2025. PMID: 40654844 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

