Vaccine efficacy of NVX-CoV2373 against SARS-CoV-2 infection in adolescents in the USA: an ancillary study to a phase 3, observer-blinded, randomised, placebo-controlled trial
- PMID: 39884302
- PMCID: PMC12383606
- DOI: 10.1016/j.lanmic.2024.100984
Vaccine efficacy of NVX-CoV2373 against SARS-CoV-2 infection in adolescents in the USA: an ancillary study to a phase 3, observer-blinded, randomised, placebo-controlled trial
Abstract
Background: Although existing COVID-19 vaccines are known to be highly effective against severe disease and death, data are needed to assess their ability to reduce SARS-CoV-2 infection. We aimed to estimate the efficacy of the NVX-CoV2373 protein subunit vaccine against SARS-CoV-2 infection, regardless of symptoms, among adolescents.
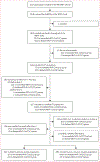
Methods: We performed an ancillary observational study (SNIFF) to the phase 3, observer-blinded, randomised, placebo-controlled PREVENT-19 trial that assessed vaccine efficacy against symptomatic COVID-19 in the USA. Participants in the PREVENT-19 trial included healthy adolescents aged 12-17 years and with no history of laboratory-confirmed SARS-CoV-2 infection. They were randomly assigned (2:1) to receive either the NVX-CoV2373 (Novavax, Gaithersburg, MD, USA) vaccine (immediate NVX-CoV2373 group) or placebo (delayed NVX-CoV2373 group) on days 0 and 21 (initial series). After 2 months, in a crossover series, participants received two doses, 21 days apart, of the intervention that they did not receive in their initial series. Participants at 47 of the PREVENT-19 sites were invited to participate in the SNIFF study and self-collect nasal swabs at home twice weekly for SARS-CoV-2 testing to assess vaccine efficacy against SARS-CoV-2 infection. This primary outcome was defined as the first identification of SARS-CoV-2 detected by RT-PCR, regardless of symptoms, with onset within 4 weeks after the second dose of the initial vaccination series until the second dose of the crossover series. Secondary outcomes were vaccine efficacy against asymptomatic and minimally symptomatic SARS-CoV-2 infection, durability of vaccine efficacy against SARS-CoV-2 infection, and durability of vaccine efficacy against asymptomatic and minimally symptomatic infections. Outcomes were analysed in the modified intention-to-treat population, which included all participants without previous SARS-CoV-2 infection and was restricted to participants enrolled within 4 weeks of the second dose of the primary (primary analysis population) or crossover (post-crossover analysis population) series. This study is registered with ClinicalTrials.gov (NCT04611802).
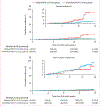
Findings: Between June 1 and Dec 17, 2021, 1196 (53·2%) of the 2247 adolescent participants recruited in the PREVENT-19 trial enrolled in the SNIFF study. The primary analysis population included 471 participants in the immediate NVX-CoV2373 group and 220 in the delayed NVX-CoV2373 group. Incidence of SARS-CoV-2 infection was 14·9 cases per 100 person-years (95% CI 7·9-25·5) in the immediate group and 54·2 cases per 100 person-years (33·6-82·9) in the delayed group; vaccine efficacy was 73·5% (95% CI 47·1-86·7; p=0·0002). Incidence of minimally symptomatic or asymptomatic SARS-CoV-2 infection was 10·3 cases per 100 person-years (95% CI 4·7-19·6) in the immediate group and 36·1 cases per 100 person-years (19·8-60·7) in the delayed group; vaccine efficacy was 72·8% (95% CI 37·1-88·2; p=0·0023). After the second crossover dose, incidence of SARS-CoV-2 was 14·6 cases per 100 person-years (95% CI 8·6-23·0) in the immediate group (receiving placebo at crossover) and 9·1 cases per 100 person-years (3·0-21·3) in the delayed group, with a durability ratio of 160·3 (95% CI 59·5-431·6; p=0·35). Almost all infections after crossover were minimally symptomatic or asymptomatic, with a durability ratio of 151·4 (55·9-410·4; p=0·41).
Interpretation: Among adolescents participating in the PREVENT-19 trial during the delta (B.1.617.2) variant wave of the COVID-19 pandemic, the NVX-CoV2373 vaccine was highly efficacious against SARS-CoV-2 infection regardless of symptoms, indicating its potential to reduce the reservoir of infections that contribute to community transmission.
Funding: US Department of Health and Human Services, Administration for Strategic Preparedness and Response, Biomedical Advanced Research and Development Authority, National Institute of Allergy and Infectious Diseases, and National Institutes of Health.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests MED received support from the National Institutes of Health (NIH)–National Institute of Allergy and Infectious Diseases (NIAID) award UM1 AI 148689, Implementing Vaccine Treatment and Evaluation Unit (VTEU) Clinical Site, through payments to her institution to conduct the SNIFF study. MED and RRR were supported by NIH Mentored Clinical Scientist Career Development Awards (K08AI170950 to MED and K08AI143923 to RRR). KLK holds grants paid to her institution from the NIH–NIAID for her role as Principal Investigator of the VTEU award; as co-chair of the PREVENT-19 trial from the Department of Health and Human Services to conduct the SNIFF study; and from Novavax for testing a COVID-19 vaccine booster. KMN reports grants paid to her institution from Pfizer, the NIH, and Vaxco for testing COVID-19 vaccines. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

