Safety and efficacy of filgotinib in patients with rheumatoid arthritis: final results of the DARWIN 3 long-term extension study
- PMID: 39884731
- PMCID: PMC11784233
- DOI: 10.1136/rmdopen-2024-004857
Safety and efficacy of filgotinib in patients with rheumatoid arthritis: final results of the DARWIN 3 long-term extension study
Abstract
Objectives: DARWIN 3 (ClinicalTrials.gov: NCT02065700) assessed the safety and efficacy of filgotinib in a long-term extension (LTE) of two phase II randomised controlled rheumatoid arthritis (RA) trials.
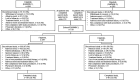
Methods: Eligible patients completing the 24-week DARWIN 1 (filgotinib plus methotrexate) and DARWIN 2 (filgotinib monotherapy) trials could enrol. Patients received filgotinib 200 mg/day, except 15 men who received filgotinib 100 mg/day. The primary endpoints were safety and tolerability, which were assessed by the incidence of treatment-emergent adverse events (TEAEs). Safety and efficacy analyses included all enrolled patients who received ≥1 dose of filgotinib in DARWIN 3.
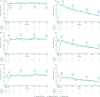
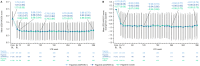
Results: 739 patients entered the LTE. The total patient-years of exposure (PYE) to filgotinib was 3706.3 years; the mean exposure duration was 259.8 weeks. 497 patients (67.3%) discontinued prematurely (including 266 TEAEs and 172 withdrawals due to the patient's decision or 'sponsor request'). Overall exposure-adjusted incidence rate (EAIR) was 67 (95% CI 62 to 72.2)/100 PYE for TEAEs and 3.8 (95% CI 3.2 to 4.5)/100 PYE for serious TEAEs. EAIR of infections was 23.3 (95% CI 21.2 to 25.6)/100 PYE, 1.3 (95% CI 0.9 to 1.7)/100 PYE for serious infections and 1.3 (95% CI 0.9 to 1.7)/100 PYE for herpes zoster. EAIRs of major adverse cardiovascular events (0.19 (95% CI 0.8 to 0.39)/100 PYE) and malignancies (0.6 (95% CI 0.4 to 0.9)/100 PYE) were low. Disease response assessed using non-responder imputation plateaued at LTE week 12 before slowly declining over time, with overall American College of Rheumatology (ACR)20/50/70 response rates of 26.9%/20.2%/14.7% at week 396.
Conclusion: Filgotinib was well tolerated in patients with RA for up to 8 years. Safety and efficacy profiles were maintained in patients previously receiving either filgotinib plus methotrexate or filgotinib monotherapy.
Keywords: Antirheumatic Agents; Arthritis; Arthritis, Rheumatoid.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RW reports consultancy fees and speaker fees from Celltrion, Galapagos and Gilead. KLW reports consultancy fees from AbbVie, AstraZeneca, BMS, Galapagos, Gilead, GSK, Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB; and grant/research support from BMS and Pfizer. AK reports consultancy fees from Amgen, AbbVie, BMS, Janssen, Novartis, Pfizer and UCB. MG reports grant/research support from AbbVie, Aclaris, Galapagos, Janssen, Lilly and Nimbus. LD reports consultancy fees from AbbVie, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, BMS, Celltrion, Galapagos, GSK, Janssen, Kiniksa Pharmaceuticals, Lilly, Novartis, Pfizer, Roche, Sanofi-Genzyme, Sobi and Takeda; and grant/research support from AbbVie, BMS, Celgene, GSK, Janssen, Kiniksa Pharmaceuticals, MSD, Mundipharma, Novartis, Pfizer, Roche, Sanofi-Genzyme and Sobi. RC none declared. RB and DdV were employees of, and shareholders in, Galapagos at the time of the study. VM is an employee of, and shareholder in, Galapagos. LHL is an employee of Alfasigma S.p.A. MCG was an employee of, and shareholder in, Gilead at the time of the analysis. PE reports consultancy fees from AbbVie, BMS, Boehringer Ingelheim, Galapagos, Gilead, Lilly and Novartis; speaker fees from AbbVie, BMS, Boehringer Ingelheim, Galapagos, Gilead, Lilly, Novartis and Samsung; and grant/research support from AbbVie, BMS, Lilly, Novartis, Roche and Samsung. PV reports consultancy fees from Alfasigma S.p.A., Boehringer Ingelheim, Cytryl, Galapagos, Lilly, Pfizer and Sidekick Health; speaker fees from AbbVie, Galapagos, Lilly, Medicongress and Roularta; and grant/research support from Galapagos and Pfizer. RA reports consultancy fees from AbbVie, Amgen, Biogen, BMS, Celltrion, Gilead, Janssen, Lilly, Medac, MSD, Mylan, Novartis, Pfizer, Roche, Sandoz, Sanofi-Genzyme, UCB and Viatris.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
