A proof-of-mechanism trial in asthma with lunsekimig, a bispecific NANOBODY molecule
- PMID: 39884759
- PMCID: PMC12018761
- DOI: 10.1183/13993003.01461-2024
A proof-of-mechanism trial in asthma with lunsekimig, a bispecific NANOBODY molecule
Abstract
Background: Monovalent biologics blocking thymic stromal lymphopoietin (TSLP) or interleukin (IL)-13 have been shown to elicit pharmacodynamic responses in asthma following a single dose. Therefore, dual blockade of these cytokines may result in an enhanced response compared to single targeting and has the potential to break efficacy ceilings in asthma. This study assessed the safety and tolerability of lunsekimig, a bispecific NANOBODY molecule that blocks TSLP and IL-13, and its effect on type 2 (T2) inflammatory biomarkers and lung function in asthma.
Methods: This was a phase 1b, single-dose (subcutaneous lunsekimig 400 mg or placebo), randomised (2:1), double-blind, proof-of-mechanism study in 36 participants with mild-to-moderate asthma and elevated exhaled nitric oxide fraction (F ENO; ≥25 ppb), a marker of airway inflammation. The primary end-point was safety and tolerability through day 71. The main pharmacodynamic secondary end-point was change from baseline in F ENO at day 29.
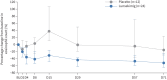
Results: Lunsekimig was well tolerated, with no serious treatment-emergent adverse events. F ENO was significantly reduced from day 8 through day 29 after a single dose, with change from baseline of -40.9 (90% CI -55.43- -26.39) ppb (p<0.0001) versus placebo at day 29. Blood-based T2 biomarkers at day 29 were significantly reduced from baseline. Lung function, particularly small airway dysfunction, was numerically improved at day 29, most notably in participants with impaired lung function at baseline.
Conclusions: A single dose of lunsekimig was well tolerated, significantly suppressed T2 inflammation and improved lung function in mild-to-moderate asthma.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: A. Deiteren, E. Krupka, L. Bontinck, K. Imberdis, G. Conickx, H.W. Staudinger and B.T. Suratt are employees of Sanofi, and may hold stock and/or stock options in the company. S. Bas has no potential conflicts of interest to disclose. N. Patel was an employee of Sanofi at the time of manuscript development, and is currently the Chief Medical Officer at CRISPR Therapeutics.
Figures




Comment in
-
Lunsekimig's bispecific targeting of IL-13 and TSLP in asthma: dual targets for synergistic effects?Eur Respir J. 2025 Apr 24;65(4):2500153. doi: 10.1183/13993003.00153-2025. Print 2025 Apr. Eur Respir J. 2025. PMID: 40274300 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
