Protein biomarkers of interstitial lung abnormalities in relatives of patients with pulmonary fibrosis
- PMID: 39884764
- PMCID: PMC12138028
- DOI: 10.1183/13993003.01349-2024
Protein biomarkers of interstitial lung abnormalities in relatives of patients with pulmonary fibrosis
Abstract
Rationale: First-degree relatives of patients with pulmonary fibrosis (referred to here as relatives) are at high risk for interstitial lung abnormalities (ILA), highlighting the need for biomarkers for risk prediction. We aimed to identify blood proteins associated with and predictive of ILA among relatives of patients with pulmonary fibrosis.
Methods: Relatives enrolled in two independent cohorts had protein levels measured using an aptamer-based proteomic platform. ILA were assessed with computed tomography scans as per Fleischner Society recommendations. Protein associations with ILA were assessed using regression. Significant proteins were used with clinical variables to detect ILA.
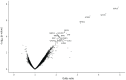
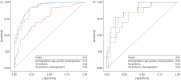
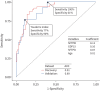
Results: Of 237 relatives from two independent cohorts, 26% had ILA. Seven proteins were associated with ILA in the discovery cohort after false discovery rate adjustment, and all remained significant after adjusting for age, gender and smoking status. Six of the seven proteins were significantly associated in the validation cohort, including growth differentiation factor 15, surfactant protein D and surfactant protein B. In a multivariable model, six proteins combined with basic demographics in the discovery cohort had an area under the curve of 0.92 (0.88 in the validation cohort). Least absolute shrinkage and selection operator modelling identified three proteins and age as predictors, with an area under the curve of 0.89 in the validation cohort. When applied to the combined cohorts, this simple model would reduce the need for computed tomography imaging in one of every three relatives screened.
Conclusion: Peripheral blood proteins are associated with ILA in relatives of patients with pulmonary fibrosis and can be used to detect them. Our findings demonstrate the potential use of blood biomarkers in this high-risk group and suggest molecular targets for future investigation.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: G.T. Axelsson reports travel support for meetings from Boehringer Ingelheim. M.B. Rice reports lecture honoraria paid by University of Southern California, University of Utah, New York University, University of North Carolina, University of Vermont and Northwestern University; payment from Conservation Law Foundation for providing an expert opinion; support for American Thoracic Society (ATS) registration in 2022 due to a role as programme committee chair; leadership of the ATS Environmental Health Policy committee until May 2020; programme committee chair 2022–2023 for the Environmental, Occupational and Population Health (EOPH) assembly of ATS; and chair-elect of the EOPH assembly of ATS 2024. J.S. Lee reports grants from Boehringer Ingelheim; consulting fees from Blade, Avalyn, Boehringer Ingelheim, United Therapeutics, AstraZeneca, Elima and Eleven P15; participation on a data safety monitoring board or advisory board for United Therapeutics (TETON trial) and Avalyn Pharma (ATLAS trial); acting as a senior medical advisor for the Pulmonary Fibrosis Foundation; and receiving a research gift from Pliant Therapeutics. H. Hatabu reports grants from Canon Medical Systems Inc. and Konica Minolta Inc. and consulting fees from Boehringer Ingelheim and Canon Medical Systems Inc. B.A. Raby reports royalties from UpToDate as an editor. D.A. Schwartz reports consulting fees from Vertex and being a founder and chief scientific officer of Eleven P15, Inc., a company focused on the early diagnosis and treatment of pulmonary fibrosis. I.O. Rosas reports grants from Boehringer Ingelheim, Genentech and Roche; and participation on an advisory board for Boehringer Ingelheim, Avalyn Pharma and Structure Therapeutics. G.M. Hunninghake reports consulting fees from Boehringer Ingelheim and Gerson Lehrman Group, and lecture fees from Boehringer Ingelheim. The remaining authors have no potential conflicts of interest to disclose.
Figures

Comment in
-
Screening relatives of familial pulmonary fibrosis patients: who, when, how and why?Eur Respir J. 2025 Jun 5;65(6):2500019. doi: 10.1183/13993003.00019-2025. Print 2025 Jun. Eur Respir J. 2025. PMID: 40473305 No abstract available.
References
MeSH terms
Substances
Grants and funding
- R01 ES031252/ES/NIEHS NIH HHS/United States
- T32 HL007633/HL/NHLBI NIH HHS/United States
- U01 TR001810/TR/NCATS NIH HHS/United States
- K08 HL173562/HL/NHLBI NIH HHS/United States
- R01 HL118455/HL/NHLBI NIH HHS/United States
- UG3 HL151865/HL/NHLBI NIH HHS/United States
- P01 HL132825/HL/NHLBI NIH HHS/United States
- UM1 TR004408/TR/NCATS NIH HHS/United States
- R01 HL149836/HL/NHLBI NIH HHS/United States
- R01 HL158668/HL/NHLBI NIH HHS/United States
- R01 HL130974/HL/NHLBI NIH HHS/United States
- U01 HL133232/HL/NHLBI NIH HHS/United States
- UH3 HL151865/HL/NHLBI NIH HHS/United States
- K08 HL140087/HL/NHLBI NIH HHS/United States
- R01 HL111024/HL/NHLBI NIH HHS/United States
- R01 HL135142/HL/NHLBI NIH HHS/United States
- I01 BX005295/BX/BLRD VA/United States
- R01 CA203636/CA/NCI NIH HHS/United States
- P01 HL162607/HL/NHLBI NIH HHS/United States
- U01 CA209414/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
