Cerebellar Development and the Burden of Prematurity
- PMID: 39885037
- PMCID: PMC11782465
- DOI: 10.1007/s12311-025-01790-6
Cerebellar Development and the Burden of Prematurity
Abstract
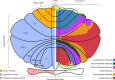
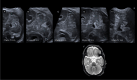
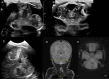
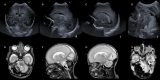
The role of the cerebellum in the neurodevelopmental outcomes of preterm infants has often been neglected. However, accumulating evidence indicates that normal cerebellar development is disrupted by prematurity-associated complications causing cerebellar injury and by prematurity itself. This hampers not only the normal development of motor skills and gait, but also cognitive, language, and behavioral development, collectively referred to as "developmental cognitive affective syndrome." In this comprehensive narrative review, we provide the results of an extensive literature search in PubMed and Embase to summarize recent evidence on altered cerebellar development in premature infants, focusing on neuroimaging findings, its causative factors and its impact on long-term neurodevelopmental outcomes.
Keywords: Cerebellar disease; Cerebellum; Neonate; Preterm infant.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interest: TM receives funding from Anna-Mueller-Grocholski Foundation for the prospective CSI NeO Study. SJS received funding from the Raynor Foundation for the Raynor Cerebellar Project. No funding has been received for this review. The authors have nothing further to disclose.
Figures



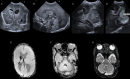
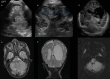
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

