GATA2 mutated allele specific expression is associated with a hyporesponsive state of HSC in GATA2 deficiency syndrome
- PMID: 39885120
- PMCID: PMC11782539
- DOI: 10.1038/s41408-025-01213-z
GATA2 mutated allele specific expression is associated with a hyporesponsive state of HSC in GATA2 deficiency syndrome
Abstract
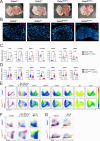
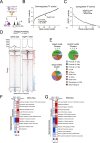
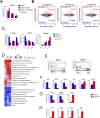
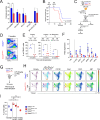
GATA2 germline mutations lead to a syndrome characterized by immunodeficiency, vascular disorders and myeloid malignancies. To elucidate how these mutations affect hematopoietic homeostasis, we created a knock-in mouse model expressing the recurrent Gata2 R396Q missense mutation. Employing molecular and functional approaches, we investigated the mutation's impact on hematopoiesis, revealing significant alterations in the hematopoietic stem and progenitor (HSPC) compartment in young age. These include increased LT-HSC numbers, reduced self-renewal potential, and impaired response to acute inflammatory stimuli. The mature HSPC compartment was primarily affected at the CMP sub-population level. In the mutant LT-HSC population, we identified an aberrant subpopulation strongly expressing CD150, resembling aging, but occurring prematurely. This population showed hyporesponsiveness, accumulated over time, and exhibited allele-specific expression (ASE) favoring the mutated Gata2 allele, also observed in GATA2 mutated patients. Our findings reveal the detrimental impact of a Gata2 recurrent missense mutation on the HSC compartment contributing to its functional decline. Defects in the CMP mature compartment, along with the inflammatory molecular signature, explain the loss of heterogeneity in HPC compartment observed in patients. Finally, our study provides a valuable model that recapitulates the ASE-related pathology observed in GATA2 deficiency, shedding light on the mechanisms contributing to the disease's natural progression.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Tsai F-Y, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–6. - PubMed
-
- Rodrigues NP. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106:477–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

