Stroma-derived Dickkopf-1 contributes to the suppression of NK cell cytotoxicity in breast cancer
- PMID: 39885132
- PMCID: PMC11782527
- DOI: 10.1038/s41467-025-56420-w
Stroma-derived Dickkopf-1 contributes to the suppression of NK cell cytotoxicity in breast cancer
Abstract
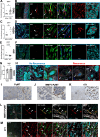
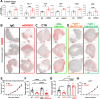
Mechanisms related to tumor evasion from NK cell-mediated immune surveillance remain enigmatic. Dickkopf-1 (DKK1) is a Wnt/β-catenin inhibitor, whose levels correlate with breast cancer progression. We find DKK1 to be expressed by tumor cells and cancer-associated fibroblasts (CAFs) in patient samples and orthotopic breast tumors, and in bone. By using genetic approaches, we find that bone-derived DKK1 contributes to the systemic DKK1 elevation in tumor-bearing female mice, while CAFs contribute to DKK1 at primary tumor site. Systemic and bone-specific DKK1 targeting reduce tumor growth. Intriguingly, deletion of CAF-derived DKK1 also limits breast cancer progression, without affecting its levels in circulation, and regardless of DKK1 expression in the tumor cells. While not directly supporting tumor proliferation, stromal-DKK1 suppresses NK cell activation and cytotoxicity by downregulating AKT/ERK/S6 phosphorylation. Importantly, increased DKK1 levels and reduced cytotoxic NK cells are detected in women with progressive breast cancer. Our findings indicate that DKK1 represents a barrier to anti-tumor immunity through suppression of NK cells.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The DKK1-neutralizing antibody mDKN01 and relevant IgG control antibody were provided at no cost for this study by Leap Therapeutics. No financial support from Leap Therapeutics was provided to perform this study. M.H. is employed by Leap Therapeutics and owns company stocks. None of the other authors have competing interests in this work.
Figures






Update of
-
Cancer-associated fibroblast-derived Dickkopf-1 suppresses NK cell cytotoxicity in breast cancer.Res Sq [Preprint]. 2024 Apr 8:rs.3.rs-4202878. doi: 10.21203/rs.3.rs-4202878/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Jan 30;16(1):1183. doi: 10.1038/s41467-025-56420-w. PMID: 38659818 Free PMC article. Updated. Preprint.
References
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin.70, 7–30 (2020). - PubMed
-
- Cortes, J. et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med.387, 217–226 (2022). - PubMed
-
- Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med.386, 556–567 (2022). - PubMed
MeSH terms
Substances
Grants and funding
- P50 CA094056/CA/NCI NIH HHS/United States
- T32 CA113275/CA/NCI NIH HHS/United States
- R01 CA217208/CA/NCI NIH HHS/United States
- R01 CA270030/CA/NCI NIH HHS/United States
- F31 CA271721/CA/NCI NIH HHS/United States
- R01 CA205239/CA/NCI NIH HHS/United States
- F31 GM146361/GM/NIGMS NIH HHS/United States
- T32 GM139774/GM/NIGMS NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 CA235096/CA/NCI NIH HHS/United States
- CA270030/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P50 CA171963/CA/NCI NIH HHS/United States
- R01 AR066551/AR/NIAMS NIH HHS/United States
- P30 AR074992/AR/NIAMS NIH HHS/United States
- AR066551/U.S. Department of Health & Human Services | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- CA235096/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- F31 CA284858/CA/NCI NIH HHS/United States
- CA091842/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 AR057235/AR/NIAMS NIH HHS/United States
- R01 AG059244/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

