Mitochondrial KMT9 methylates DLAT to control pyruvate dehydrogenase activity and prostate cancer growth
- PMID: 39885202
- PMCID: PMC11782658
- DOI: 10.1038/s41467-025-56492-8
Mitochondrial KMT9 methylates DLAT to control pyruvate dehydrogenase activity and prostate cancer growth
Abstract
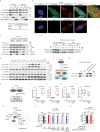
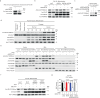
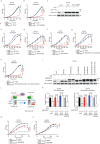
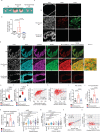
Prostate cancer (PCa) growth depends on de novo lipogenesis controlled by the mitochondrial pyruvate dehydrogenase complex (PDC). In this study, we identify lysine methyltransferase (KMT)9 as a regulator of PDC activity. KMT9 is localized in mitochondria of PCa cells, but not in mitochondria of other tumor cell types. Mitochondrial KMT9 regulates PDC activity by monomethylation of its subunit dihydrolipoamide transacetylase (DLAT) at lysine 596. Depletion of KMT9 compromises PDC activity, de novo lipogenesis, and PCa cell proliferation, both in vitro and in a PCa mouse model. Finally, in human patients, levels of mitochondrial KMT9 and DLAT K596me1 correlate with Gleason grade. Together, we present a mechanism of PDC regulation and an example of a histone methyltransferase with nuclear and mitochondrial functions. The dependency of PCa cells on mitochondrial KMT9 allows to develop therapeutic strategies to selectively fight PCa.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Willems, P. H., Rossignol, R., Dieteren, C. E., Murphy, M. P. & Koopman, W. J. Redox homeostasis and mitochondrial dynamics. Cell Metab.22, 207–218 (2015). - PubMed
-
- Bader, D. A. & McGuire, S. E. Tumour metabolism and its unique properties in prostate adenocarcinoma. Nat. Rev. Urol.17, 214–231 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

