Biofabrication of zinc oxide nanoparticles using Moringa oleifera, characterization and statistical optimization for their application in crystal violet adsorption
- PMID: 39885265
- PMCID: PMC11782614
- DOI: 10.1038/s41598-025-86629-0
Biofabrication of zinc oxide nanoparticles using Moringa oleifera, characterization and statistical optimization for their application in crystal violet adsorption
Abstract
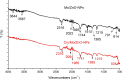
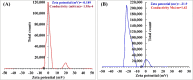
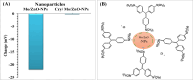
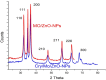
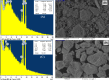
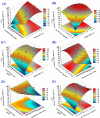
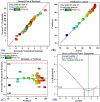
Crystal violet (Cry) is an essential textile dye belonging to the triphenylmethane group, that is widely used in the textile industry. It is also applied for paper printing and Gram staining. Previously, it was significant as a topical antiseptic due to its antibacterial, antifungal, and anthelmintic properties. Despite its various applications, crystal violet has been recognized as a biohazard dye due to its toxic and carcinogenic properties. It persists in the environment with long-lasting effects and has detrimental impacts. In this research, water extract from Moringa oleifera leaves is employed as environmentally friendly methods to synthesize zinc oxide nanoparticles (Mo/ZnO-NPs), and characterized by TEM, EDX, FT-IR, and Zeta potential. Mo/ZnO-NPs exhibit a Zeta potential of - 21.9 mV, and X-ray diffraction (XRD) analysis confirms their crystallographic structure. The size of the biogenic Mo/ZnO-NPs ranges from 5.52 to 41.59 nm. This study was designed to estimate and maximize the ability of Mo/ZnO-NPs to remove crystal violet using Central Composite Design (CCD), considering pH (ranging from 3 to 11), incubation time (ranging from 30 to 150), nanoparticles concentrations (ranging from 0.2 to 1.8 mg/mL), and crystal violet concentrations (ranging from 25 to 125 ppm). The maximum percentage value of removal of crystal violet by Mo/ZnO-NPs was 97.26 with optimal conditions of pH 9, incubation time 120 min, Mo/ZnO-NPs 1.4 mg/mL, and crystal violet concentration of 50 ppm. The best-predicted conditions that caused the highest removal of crystal violet (97.8%) were determined using the desirability function as pH 10, incubation time of 140 min, Mo/ZnO-NPs concentrations of 1.3 mg/mL, and a concentration of crystal violet of 80 ppm. Under these optimal conditions, the maximum experimental crystal violet removal% by Mo/ZnO-NPs was (98.7%) was verified. Mo/ZnO-NPs synthesized by Moringa oleifera can be a promising candidate for the adsorption of crystal violet.
Keywords: Moringa oleifera; Central composite design; Crystal violet removal; ZnO-NPs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Green Synthesis of Carbon Nanodots (CNDs) Moderated by Flavonoid Extracts from Moringa oleifera Leaves and Co-Doped Sulfur/Nitrogen (NS - CNDs - Fla) and Their Potential for Heavy Metals Sensing Application.J Fluoresc. 2025 Jul;35(7):5603-5615. doi: 10.1007/s10895-024-03931-2. Epub 2024 Sep 25. J Fluoresc. 2025. PMID: 39320631
-
Phytogenic nanoparticles: synthesis, characterization, and their roles in physiology and biochemistry of plants.Biometals. 2024 Feb;37(1):23-70. doi: 10.1007/s10534-023-00542-5. Epub 2023 Nov 2. Biometals. 2024. PMID: 37914858 Review.
-
Antimicrobial potential of floral extract-decorated nanoparticles against food-borne pathogens.Discov Nano. 2025 Jul 5;20(1):103. doi: 10.1186/s11671-025-04292-w. Discov Nano. 2025. PMID: 40617998 Free PMC article.
-
Thermo stable ZnO NPs/Asiatic acid nanocomposites for acidogenic neutralization, anti-biofilm, and enamel protection in dental enamel reinforcement.J Dent. 2025 Oct;161:106001. doi: 10.1016/j.jdent.2025.106001. Epub 2025 Jul 25. J Dent. 2025. PMID: 40716557
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Anti-biofouling adsorptive sheet based on polyethersulfone/ dried algal biomass / ZnO nanoparticles for dyes removal.Sci Rep. 2025 May 21;15(1):17599. doi: 10.1038/s41598-025-02081-0. Sci Rep. 2025. PMID: 40399398 Free PMC article.
References
-
- Rafiq, A. et al. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem.97, 111–128. 10.1016/J.JIEC.2021.02.017 (2021).
-
- Garg, A. & Chopra, L. Dye Waste: A significant environmental hazard. Mater. Today Proc.48, 1310–1315. 10.1016/J.MATPR.2021.09.003 (2022).
-
- Benkhaya, S., M’rabet, S., Lgaz, H., El Bachiri, A. & El Harfi, A. Dyes: classification, pollution, and environmental effects. Dye Biodegrad. Mech. Tech. Recent Adv.1, 50. 10.1007/978-981-16-5932-4_1 (2022).
-
- Kishor, R. et al. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng.9(2), 105012 (2021).
-
- Ismail, M. et al. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Design25(34), 3645–3663. 10.2174/1381612825666191021142026 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

