Plasma proteomic evidence for increased β-amyloid pathology after SARS-CoV-2 infection
- PMID: 39885359
- PMCID: PMC11922756
- DOI: 10.1038/s41591-024-03426-4
Plasma proteomic evidence for increased β-amyloid pathology after SARS-CoV-2 infection
Abstract
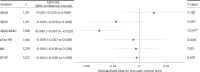
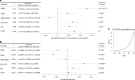
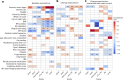
Previous studies have suggested that systemic viral infections may increase risks of dementia. Whether this holds true for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus infections is unknown. Determining this is important for anticipating the potential future incidence of dementia. To begin to do this, we measured plasma biomarkers linked to Alzheimer's disease pathology in the UK Biobank before and after serology-confirmed SARS-CoV-2 infections. SARS-CoV-2 infection was associated with biomarkers associated with β-amyloid pathology: reduced plasma Aβ42:Aβ40 ratio and, in more vulnerable participants, lower plasma Aβ42 and higher plasma pTau-181. The plasma biomarker changes were greater in participants who had been hospitalized with COVID-19 or had reported hypertension previously. We showed that the changes in biomarkers were linked to brain structural imaging patterns associated with Alzheimer's disease, lower cognitive test scores and poorer overall health evaluations. Our data from this post hoc case-control matched study thus provide observational biomarker evidence that SARS-CoV-2 infection can be associated with greater brain β-amyloid pathology in older adults. While these results do not establish causality, they suggest that SARS-CoV-2 (and possibly other systemic inflammatory diseases) may increase the risk of future Alzheimer's disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests. P.M.M. is a consultant for Biogen, Sudo Therapeutics, Nimbus, Astex, GSK and Sangamo. He received research funding for aspects of this work from Biogen and the UK DRI. P.M.M. has also received research funding unrelated to this work from Biogen and Bristol Meyers Squibb. H.R. and B.B.S. were full-time employees at Biogen during the data generation for this study. B.B.S. is an employee of Bristol Myers Squibb. H.R. is an employee at insitro Inc. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly and Roche; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. C.D.W. is an employee of Johnson & Johnson Innovative Medicine. No organizations listed here made any contributions to the conceptualization or preparation of this study beyond the disclosed individual contributions of authors who were employees. They are listed only for the potential perception of competing interests associated with drug or biomarker development. The other authors declare no competing interests.
Figures



References
-
- Ou, Y.-N. et al. Associations of infectious agents with Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis.75, 299–309 (2020). - PubMed
-
- Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A69, S4–S9 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

