Decoding pan-cancer treatment outcomes using multimodal real-world data and explainable artificial intelligence
- PMID: 39885364
- PMCID: PMC11864985
- DOI: 10.1038/s43018-024-00891-1
Decoding pan-cancer treatment outcomes using multimodal real-world data and explainable artificial intelligence
Abstract
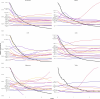
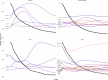
Despite advances in precision oncology, clinical decision-making still relies on limited variables and expert knowledge. To address this limitation, we combined multimodal real-world data and explainable artificial intelligence (xAI) to introduce AI-derived (AID) markers for clinical decision support. We used xAI to decode the outcome of 15,726 patients across 38 solid cancer entities based on 350 markers, including clinical records, image-derived body compositions, and mutational tumor profiles. xAI determined the prognostic contribution of each clinical marker at the patient level and identified 114 key markers that accounted for 90% of the neural network's decision process. Moreover, xAI enabled us to uncover 1,373 prognostic interactions between markers. Our approach was validated in an independent cohort of 3,288 patients with lung cancer from a US nationwide electronic health record-derived database. These results show the potential of xAI to transform the assessment of clinical variables and enable personalized, data-driven cancer care.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: V.G. receives honoraria from Bristol Myers Squibb, Pfizer, Ipsen, Eisai, Merck Sharp & Dohme (MSD) Oncology, Merck HealthCare, EUSAPharm, Apogepha and Ono Pharmaceutical; has an advisory role at BMS, Pfizer, MSD Oncology, Merck HealthCare, Ipsen, Eisai, Debiopharm, PCI Biotech, Cureteq and Oncorena; and received travel funding from Pfizer, Ipsen and Merck HealthCare. B.H. has an advisory role at ABX, AAA/Novartis, Astellas, AstraZeneca, Bayer, BMS, Janssen R&D, Lightpoint Medical and Pfizer; receives research funding from Astellas, BMS, AAA/Novartis, German Research Foundation, Janssen R&D and Pfizer; and receives travel funding from Astellas, AstraZeneca, Bayer and Janssen. D.S. receives personal fees for advisory boards of BMS, Immunocore, MSD, Neracare, Novartis, Pfizer, Philogen, Pierre Fabre, Sanofi and Regeneron; personal fees as an invited speaker from BMS, Merck Serono, MSD, Novartis, Roche and Sanofi; personal fees (financial interest) for steering committee membership from BMS and MSD; personal support (no financial interest) for steering committee membership from Novartis; institutional support as a coordinating principal investigator (no financial interest) from BMS, MSD, Novartis and Pierre Fabre; institutional support as a local principal investigator (no financial interest) from Philogen and Sanofi; institutional research grant support (financial interest) from BMS and MSD; and is an EORTC-MG Member of the Board of Directors (no financial interest). J.T.S. receives honoraria as consultant or for continuing medical education presentations from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Immunocore, MSD Sharp Dohme, Novartis, Roche/Genentech and Servier; his institution receives research funding from Abalos Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eisbach Bio and Roche/Genentech; and he holds ownership and serves on the Board of Directors of Pharma15, all outside the submitted work. M.T. receives speaker fees and personal support from AstraZeneca, Daiichi Sankyo, Novartis, Bayer, Asklepios and Edwards LifeSciences. M.W. receives honoraria and has an advisory role: Amgen, AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Janssen, Novartis, Pfizer, Roche, Takeda. Research funding: Bristol-Myers Squibb, Takeda. M.S. is a consultant (compensated) for Amgen, AstraZeneca, Blueprint Medicines, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Roche, Sanofi and Takeda; receives honoraria for CME presentations from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, MSD, Novartis, Roche and Sanofi; and receives research funding (institutional) from AstraZeneca and Bristol-Myers Squibb. K.-R.M., F.K. and G.M. hold patents related to this work (9558550; 20180018553) and are co-founders of the computational pathology start-up Aignostics, Berlin. The remaining authors declare no competing interests related to this study.
Figures















References
MeSH terms
Substances
Grants and funding
- FU 356/12-2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 418179183 - KFO 337(RO 3577/3-2 (AR), RO 3577/7-1 (AR), SCHA 422/17-1 (DS)/Deutsche Forschungsgemeinschaft (German Research Foundation)
- BMBF; 01KD2206A/SATURN3/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01IS14013A-E, 01GQ1115, 01GQ0850, 01IS18056A, 01IS18025A and 01IS18037A/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
LinkOut - more resources
Full Text Sources
Medical

