Targeting PCSK9, through an innovative cVLP-based vaccine, enhanced the therapeutic activity of a cVLP-HER2 vaccine in a preclinical model of HER2-positive mammary carcinoma
- PMID: 39885551
- PMCID: PMC11784117
- DOI: 10.1186/s12967-025-06126-w
Targeting PCSK9, through an innovative cVLP-based vaccine, enhanced the therapeutic activity of a cVLP-HER2 vaccine in a preclinical model of HER2-positive mammary carcinoma
Abstract
Background: HER2-targeted therapies have revolutionized the treatment of HER2-positive breast cancer patients, leading to significant improvements in tumor response rates and survival. However, resistance and incomplete response remain considerable challenges. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition is a novel therapeutic strategy for the management of dyslipidemia by enhancing the clearance of low-density lipoprotein cholesterol receptors, however recent evidence also shows links between PCSK9 and cancer cells. We present an innovative immunization approach combining capsid virus-like particle (cVLP)-based vaccines against HER2 and PCSK9.
Methods: The therapeutic activity of the combined vaccine was evaluated in female mice challenged with HER2-positive mammary carcinoma cells. Controls included untreated mice and mice treated with cVLP-PCSK9 and cVLP-HER2 as standalone therapies. Antibodies elicited by vaccinations were detected through ELISA immunoassay. The functional activity of the antibodies was tested in 3D-soft agar assay on human HER2 + + + trastuzumab sensitive and resistant cells.
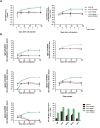
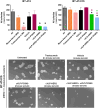
Results: Mice vaccinated with cVLP-HER2 + cVLP-PCSK9 displayed tumor regression from the 40th day after cell challenge in 100% of mice remaining tumor-free even 4 months later. In contrast, 83% of mice treated with cVLP-HER2 vaccine alone experienced an initial tumor regression, followed by tumor relapse in 60% of subjects. Untreated mice and mice treated with the cVLP-PCSK9 vaccine alone developed progressive tumors within 1-2 months after cell injection. The combined vaccine approach elicited strong anti-human HER2 antibody responses (reaching 1-2 mg/ml range) comprising multiple immunoglobulins isotypes. cVLP-PCSK9 vaccine elicited anti-PCSK9 antibody responses, resulting in a marked reduction in PCSK9 serum levels. Although the anti-PCSK9 response was reduced when co-administered with cVLP-HER2, it remained significant. Moreover, both cVLP-HER2 + cVLP-PCSK9 and cVLP-HER2 alone induced anti-HER2 antibodies able to inhibit the 3D growth of human HER2 + + + BT-474 and trastuzumab-resistant BT-474 C5 cells. Strikingly, antibodies elicited by the combined vaccination were more effective than those elicited by the cVLP-HER2 vaccine alone in the inhibition of trastuzumab-resistant C5 cells.
Conclusions: The results indicate that cVLP-PCSK9 vaccination shows adjuvant activity when combined with cVLP-HER2 vaccine, enhancing its therapeutic efficacy against HER2-positive breast cancer and holding promise in overcoming the challenges posed by resistance and incomplete responses to HER2-targeted therapy.
Keywords: Breast cancer; Cancer progression; HER2; PCSK9; Therapeutic cancer vaccines; Therapy resistance; Virus-like particle (VLP)-based vaccine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All in vivo experiments were performed according to Italian and European laws and were authorized by the Italian Ministry of Health (letter 714–2017-PR). Consent for Publication: Not applicable. Competing interests: L.G., C.F. and A.F.S. are listed as co-inventors on a patent application covering the Delivery of a cVLP-based modular vaccine platform in a nucleic acid platform (P5856PC00). Employees of AdaptVac (L.G., C.F. and A.F.S.), a company commercializing virus-like particle display technology and vaccine, including several patents.
Figures


Similar articles
-
Sequential Anti-PD1 Therapy Following Dendritic Cell Vaccination Improves Survival in a HER2 Mammary Carcinoma Model and Identifies a Critical Role for CD4 T Cells in Mediating the Response.Front Immunol. 2019 Aug 14;10:1939. doi: 10.3389/fimmu.2019.01939. eCollection 2019. Front Immunol. 2019. PMID: 31475002 Free PMC article.
-
Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system.Atherosclerosis. 2019 Apr;283:69-78. doi: 10.1016/j.atherosclerosis.2019.02.001. Epub 2019 Feb 14. Atherosclerosis. 2019. PMID: 30797988
-
Vaccination with human anti-trastuzumab anti-idiotype scFv reverses HER2 immunological tolerance and induces tumor immunity in MMTV.f.huHER2(Fo5) mice.Breast Cancer Res. 2011 Feb 4;13(1):R17. doi: 10.1186/bcr2826. Breast Cancer Res. 2011. PMID: 21294885 Free PMC article.
-
Vaccine strategies for lowering LDL by immunization against proprotein convertase subtilisin/kexin type 9.Curr Opin Lipidol. 2016 Aug;27(4):345-50. doi: 10.1097/MOL.0000000000000312. Curr Opin Lipidol. 2016. PMID: 27389630 Review.
-
HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development.Arch Immunol Ther Exp (Warsz). 2020 Jan 9;68(1):2. doi: 10.1007/s00005-019-00566-1. Arch Immunol Ther Exp (Warsz). 2020. PMID: 31915932 Free PMC article. Review.
Cited by
-
PCSK9 expression and cancer survival: a prognostic biomarker at the intersection of oncology and geroscience.Geroscience. 2025 Jun 27. doi: 10.1007/s11357-025-01733-3. Online ahead of print. Geroscience. 2025. PMID: 40576911
References
-
- Valenza C, Guidi L, Battaiotto E, Trapani D, Sartore Bianchi A, Siena S, Curigliano G. Targeting HER2 heterogeneity in breast and gastrointestinal cancers. Trends Cancer. 2024;10:113–23. 10.1016/j.trecan.2023.11.001. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

