Lewy body diseases and the gut
- PMID: 39885558
- PMCID: PMC11783828
- DOI: 10.1186/s13024-025-00804-5
Lewy body diseases and the gut
Abstract
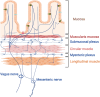
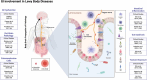
Gastrointestinal (GI) involvement in Lewy body diseases (LBDs) has been observed since the initial descriptions of patients by James Parkinson. Recent experimental and human observational studies raise the possibility that pathogenic alpha-synuclein (⍺-syn) might develop in the GI tract and subsequently spread to susceptible brain regions. The cellular and mechanistic origins of ⍺-syn propagation in disease are under intense investigation. Experimental LBD models have implicated important contributions from the intrinsic gut microbiome, the intestinal immune system, and environmental toxicants, acting as triggers and modifiers to GI pathologies. Here, we review the primary clinical observations that link GI dysfunctions to LBDs. We first provide an overview of GI anatomy and the cellular repertoire relevant for disease, with a focus on luminal-sensing cells of the intestinal epithelium including enteroendocrine cells that express ⍺-syn and make direct contact with nerves. We describe interactions within the GI tract with resident microbes and exogenous toxicants, and how these may directly contribute to ⍺-syn pathology along with related metabolic and immunological responses. Finally, critical knowledge gaps in the field are highlighted, focusing on pivotal questions that remain some 200 years after the first descriptions of GI tract dysfunction in LBDs. We predict that a better understanding of how pathophysiologies in the gut influence disease risk and progression will accelerate discoveries that will lead to a deeper overall mechanistic understanding of disease and potential therapeutic strategies targeting the gut-brain axis to delay, arrest, or prevent disease progression.
Keywords: Dementia with Lewy bodies; Gastrointestinal tract; Immunity; Inflammation; Microbiome; Parkinson’s disease; Parkinson’s disease dementia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors give their consent to publish. Competing interests: T.R.S. is a listed inventor on institutionally-assigned IP surrounding the microbiome and PD. M.G.T. has served as a member of The Michael J. Fox Foundation Scientific Advisory Board and serves on the Parkinson’s Foundation Scientific Advisory Board, the Weston Family Foundation Advisory Board, the Alzheimer’s Association Medical and Scientific Advisory Group, and as advisor for NysnoBio, IMMvention, Ventus, iMetabolic Pharma, Longevity Inc, Merck, NovoNordisk, Forward Therapeutics, Jaya, Longeveron, and Alnylam. She has received grants from MJFF/ASAP, Parkinson’s Foundation, and the National Institutes of Health (NIH). She is Editor-in-Chief for Nature Partner Journal Parkinson’s Disease, and serves as Associate Editor for Science Advances, Alzheimer’s & Dementia: Translational Research and Clinical Interventions, and the Journal of Neuroinflammation. A.B.W. has served as a member of The Michael J. Fox Executive Foundation (MJFF) Scientific Advisory Board and is a paid consultant for SciNeuro, Inc, and has been a paid consultant for EscapeBio Inc.; and has received research grants from Biogen Inc. and EscapeBio, Inc., as well as MJFF, ASAP Foundation, Parkinson’s Foundation, and the National Institutes of Health (NIH). A.B.W. is part owner of a series of LRRK2 kinase inhibitors (WO 2013166276) and part owner of induced-pluripotent stem cell lines of early-onset PD distributed by Cedars Sinai. R.A.L. receives authorship royalties from UpToDate.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

