Enhancing neural stem cell integration in the injured spinal cord through targeted PTEN modulation
- PMID: 39885671
- PMCID: PMC12407558
- DOI: 10.4103/NRR.NRR-D-24-00455
Enhancing neural stem cell integration in the injured spinal cord through targeted PTEN modulation
Abstract
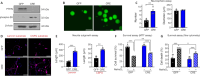
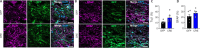
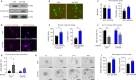
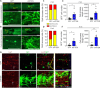
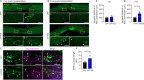
JOURNAL/nrgr/04.03/01300535-202604000-00039/figure1/v/2025-06-30T060627Z/r/image-tiff Spinal cord injury results in permanent loss of neurological functions due to severance of neural networks. Transplantation of neural stem cells holds promise to repair disrupted connections. Yet, ensuring the survival and integration of neural stem cells into the host neural circuit remains a formidable challenge. Here, we investigated whether modifying the intrinsic properties of neural stem cells could enhance their integration post-transplantation. We focused on phosphatase and tensin homolog (PTEN), a well-characterized tumor suppressor known to critically regulate neuronal survival and axonal regeneration. By deleting Pten in mouse neural stem cells, we observed increased neurite outgrowth and enhanced resistance to neurotoxic environments in culture. Upon transplantation into injured spinal cords, Pten-deficient neural stem cells exhibited higher survival and more extensive rostrocaudal distribution. To examine the potential influence of partial PTEN suppression, rat neural stem cells were treated with short hairpin RNA targeting PTEN, and the PTEN knockdown resulted in significant improvements in neurite growth, survival, and neurosphere motility in vitro . Transplantation of shPTEN-treated neural stem cells into the injured spinal cord also led to an increase in graft survival and migration to an extent similar to that of complete deletion. Moreover, PTEN suppression facilitated neurite elongation from NSC-derived neurons migrating from the lesion epicenter. These findings suggest that modifying intrinsic signaling pathways, such as PTEN, within neural stem cells could bolster their therapeutic efficacy, offering potential avenues for future regenerative strategies for spinal cord injury.
Keywords: PTEN; graft axon growth; graft survival; neural stem cell; regeneration; spinal cord injury; transplantation.
Copyright © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures

References
-
- Anderson MA, O’Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, Wollenberg AL, Kawaguchi R, Coppola G, Wang C, Deming TJ, He Z, Courtine G, Sofroniew MV. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. - PMC - PubMed
-
- Antonios JP, Farah GJ, Cleary DR, Martin JR, Ciacci JD, Pham MH. Immunosuppressive mechanisms for stem cell transplant survival in spinal cord injury. Neurosurg Focus. 2019;46:E9. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

