Memory Decline and Aberration of Synaptic Proteins in X-Linked Moesin Knockout Male Mice
- PMID: 39885788
- PMCID: PMC11788833
- DOI: 10.30773/pi.2024.0186
Memory Decline and Aberration of Synaptic Proteins in X-Linked Moesin Knockout Male Mice
Abstract
Objective: This study aims to investigate may moesin deficiency resulted in neurodevelopmental abnormalities caused by negative impact on synaptic signaling ultimately leading to synaptic structure and plasticity.
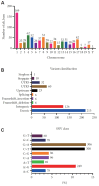
Methods: Behavioral assessments measured neurodevelopment (surface righting, negative geotaxis, cliff avoidance), anxiety (open field test, elevated plus maze test), and memory (passive avoidance test, Y-maze test) in moesin-knockout mice (KO) compared to wild-type mice (WT). Whole exome sequencing (WES) of brain (KO vs. WT) and analysis of synaptic proteins were performed to determine the disruption of signal pathways downstream of moesin. Risperidone, a therapeutic agent, was utilized to reverse the neurodevelopmental aberrance in moesin KO.
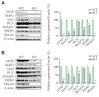
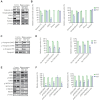
Results: Moesin-KO pups exhibited decrease in the surface righting ability on postnatal day 7 (p<0.05) and increase in time spent in the closed arms (p<0.01), showing increased anxiety-like behavior. WES revealed mutations in pathway aberration in neuron projection, actin filament-based processes, and neuronal migration in KO. Decreased cell viability (p<0.001) and expression of soluble NSF adapter protein 25 (SNAP25) (p<0.001) and postsynaptic density protein 95 (PSD95) (p<0.01) was observed in days in vitro 7 neurons. Downregulation of synaptic proteins, and altered phosphorylation levels of Synapsin I, mammalian uncoordinated 18 (MUNC18), extracellular signal-regulated kinase (ERK), and cAMP response element-binding protein (CREB) was observed in KO cortex and hippocampus. Risperidone reversed the memory impairment in the passive avoidance test and the spontaneous alternation percentage in the Y maze test. Risperidone also restored the reduced expression of PSD95 (p<0.01) and the phosphorylation of Synapsin at Ser605 (p<0.05) and Ser549 (p<0.001) in the cortex of moesin-KO.
Conclusion: Moesin deficiency leads to neurodevelopmental delay and memory decline, which may be caused through altered regulation in synaptic proteins and function.
Keywords: Memory; Moesin; Neurodevelopment; PSD95; Risperidone; Synapsin.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures




Similar articles
-
Developmental deficits, synapse and dendritic abnormalities in a Clcn4 KO autism mice model: endophenotypic target for ASD.Transl Psychiatry. 2025 Jan 25;15(1):28. doi: 10.1038/s41398-024-03201-6. Transl Psychiatry. 2025. PMID: 39863599 Free PMC article.
-
LT-102, an AMPA receptor potentiator, alleviates depression-like behavior and synaptic plasticity impairments in prefrontal cortex induced by sleep deprivation.J Affect Disord. 2024 Dec 15;367:18-30. doi: 10.1016/j.jad.2024.08.176. Epub 2024 Aug 29. J Affect Disord. 2024. PMID: 39214374
-
Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory.J Neurosci. 2019 Apr 10;39(15):2792-2809. doi: 10.1523/JNEUROSCI.1970-18.2019. Epub 2019 Feb 6. J Neurosci. 2019. PMID: 30728170 Free PMC article.
-
Disruption of Extracellular Signal-Regulated Kinase Partially Mediates Neonatal Isoflurane Anesthesia-Induced Changes in Dendritic Spines and Cognitive Function in Juvenile Mice.Int J Mol Sci. 2025 Jan 24;26(3):981. doi: 10.3390/ijms26030981. Int J Mol Sci. 2025. PMID: 39940749 Free PMC article.
-
Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D.Neuropharmacology. 2014 Feb;77:120-30. doi: 10.1016/j.neuropharm.2013.09.015. Epub 2013 Sep 22. Neuropharmacology. 2014. PMID: 24067928
References
-
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103(Pt 1):131–143. - PubMed
-
- Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

