The behavioural consequences of dystrophinopathy
- PMID: 39885828
- PMCID: PMC11911635
- DOI: 10.1242/dmm.052047
The behavioural consequences of dystrophinopathy
Abstract
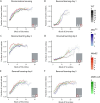
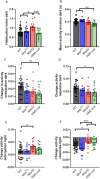
Duchenne muscular dystrophy is a severe neuromuscular disorder, caused by mutations in the DMD gene. Normally, the DMD gene gives rise to many dystrophin isoforms, of which multiple are expressed in the brain. The location of the mutation determines the number of dystrophin isoforms affected, and the absence thereof leads to behavioral and cognitive impairments. Even though behavioral studies have thoroughly investigated the effects of the loss of Dp427, and to a lesser extent of Dp140, in mice, direct comparisons between models lacking multiple dystrophin isoforms are sparse. Furthermore, a behavioral characterization of the DMD-null mouse, which lacks all dystrophin isoforms, has never been undertaken. Using a wide variety of behavioral tests, we directly compared impairments between mdx5cv, mdx52 and DMD-null mice. We confirmed the role of Dp427 in emotional reactivity. We did not find any added effects of loss of Dp140 on fear, but showed the involvement of Dp140 in spontaneous behavior, specifically in habituation and activity changes due to light/dark switches. Lastly, our results indicate that Dp71/Dp40 play an important role in many behavioral domains, including anxiety and spontaneous behavior.
Keywords: Mdx52 and DMD-null; Mdx5cv; Anxiety; Cognition; Dystrophin; Learning; Social interaction; Spontaneous behavior.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





References
-
- Alexander, M., Gasperini, M., Tsai, P., Gibbs, D., Spinazzola, J., Marshall, J., Feyder, M., Pletcher, M., Chekler, E. P. and Morris, C. (2016). Reversal of neurobehavioral social deficits in dystrophic mice using inhibitors of phosphodiesterases PDE5A and PDE9A. Transl. Psychiatry 6, e901-e901. 10.1038/tp.2016.174 - DOI - PMC - PubMed
-
- Araki, E., Nakamura, K., Nakao, K., Kameya, S., Kobayashi, O., Nonaka, I., Kobayashi, T. and Katsuki, M. (1997). Targeted disruption of exon 52 in the mouse dystrophin gene induced muscle degeneration similar to that observed in Duchenne muscular dystrophy. Biochem. Biophy. Res. Commun. 238, 492-497. 10.1006/bbrc.1997.7328 - DOI - PubMed
-
- Barboni, M. T. S., Liber, A. M. P., Joachimsthaler, A., Saoudi, A., Goyenvalle, A., Rendon, A., Roger, J. E., Ventura, D. F., Kremers, J. and Vaillend, C. (2021). Altered visual processing in the mdx52 mouse model of Duchenne muscular dystrophy. Neurobiol. Dis. 152, 105288. 10.1016/j.nbd.2021.105288 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

