Comparative gastrointestinal organoid models across species: A Zoobiquity approach for precision medicine
- PMID: 39885871
- PMCID: PMC11779682
- DOI: 10.1016/j.reth.2024.12.013
Comparative gastrointestinal organoid models across species: A Zoobiquity approach for precision medicine
Abstract
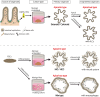
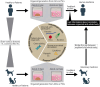
Gastrointestinal (GI) health underpins systemic well-being, yet the complexity of gut physiology poses significant challenges to understanding disease mechanisms and developing effective, personalized therapies. Traditional models often fail to capture the intricate interplay between epithelial, mesenchymal, immune, and neuronal cells that govern gut homeostasis and disease. Over the past five years, advances in organoid technology have created physiologically relevant, three-dimensional GI models that replicate native tissue architecture and function. These models have revolutionized the study of autoimmune disorders, homeostatic dysfunction, and pathogen infections, such as norovirus and Salmonella, which affect millions of humans and animals globally. In this review, we explore how organoids, derived from intestinal and pluripotent stem cells, are transforming our understanding of GI development, disease etiology, and therapeutic innovation. Through the "Zoobiquity" paradigm and "One Health" framework, we highlight the integration of companion animal organoids, which provide invaluable insights into shared disease mechanisms and preclinical therapeutic development. Despite their promise, challenges remain in achieving organoid maturation, expanding immune and neuronal integration, and bridging the gap between organoid responses and in vivo outcomes. By refining these cutting-edge platforms, we can advance human and veterinary medicine alike, fostering a holistic approach to health and disease.
Keywords: Companion animal models; Comparative biology; Disease modeling; Gastrointestinal organoids; Intestinal stem cells; Zoobiquity; iPS cells.
© 2024 The Author(s).
Conflict of interest statement
The authors have no relevant conflicts of interest to declare.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

