Successful treatment of a chronic myeloid leukemia patient with extreme thrombocytosis by a combination of imatinib and interferon‑α: A case report
- PMID: 39885917
- PMCID: PMC11775754
- DOI: 10.3892/etm.2025.12800
Successful treatment of a chronic myeloid leukemia patient with extreme thrombocytosis by a combination of imatinib and interferon‑α: A case report
Abstract
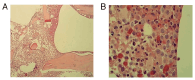
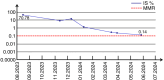
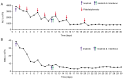
Chronic myeloid leukemia with extreme thrombocytosis (CML-T), defined by a platelet count >1,000x109/l is a rare leukemia subtype. The present case report described a 66-year-old female CML-T patient presenting with a platelet count of 3,798x109/l, but a consistently normal spleen size. Following treatment with imatinib combined with interferon-α, the patient achieved hematological remission within 2 months, with a platelet count reduction to 311x109/l and complete cytogenetic remission after 10 months. The patient experienced myocardial infarction and liver injury during treatment, which was managed with supportive care. The present case suggested that imatinib combined with interferon-α may be a safe and effective treatment option for patients with CML-T and extreme thrombocytosis and suboptimal response to imatinib monotherapy.
Keywords: chronic myeloid leukemia with thrombocytosis; imatinib; interferon-α; thrombocytosis.
Copyright: © 2025 Jia et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Long-Term Molecular Remission after Treatment with Imatinib in a Chronic Myeloid Leukemia Patient with Extreme Thrombocytosis Harboring Rare e14a3 (b3a3) BCR::ABL1 Transcript: A Case Report.Curr Oncol. 2022 Oct 28;29(11):8171-8179. doi: 10.3390/curroncol29110645. Curr Oncol. 2022. PMID: 36354705 Free PMC article.
-
Management of chronic myeloid leukemia presenting with isolated thrombocytosis and complex Philadelphia chromosome: A case report.Medicine (Baltimore). 2021 Sep 3;100(35):e27134. doi: 10.1097/MD.0000000000027134. Medicine (Baltimore). 2021. PMID: 34477162 Free PMC article.
-
Analysis of clinical characteristics and efficacy of chronic myeloid leukemia onset with extreme thrombocytosis in the era of tyrosine kinase inhibitors.Onco Targets Ther. 2017 Jul 17;10:3515-3520. doi: 10.2147/OTT.S142587. eCollection 2017. Onco Targets Ther. 2017. PMID: 28761360 Free PMC article.
-
Treatment options for chronic myeloid leukemia: imatinib versus interferon versus allogeneic transplant.Curr Opin Oncol. 2004 Mar;16(2):95-9. doi: 10.1097/00001622-200403000-00002. Curr Opin Oncol. 2004. PMID: 15075898 Review.
-
[Isolated thrombocytosis in chronic myeloid leukemia without significant leukocytosis].Rinsho Ketsueki. 2017;58(7):766-771. doi: 10.11406/rinketsu.58.766. Rinsho Ketsueki. 2017. PMID: 28781272 Review. Japanese.
References
-
- Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, et al. International consensus classification of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical and genomic data. Blood. 2022;140:1200–1228. doi: 10.1182/blood.2022015850. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
