Hydrogel inspired by "adobe" with antibacterial and antioxidant properties for diabetic wound healing
- PMID: 39885943
- PMCID: PMC11780960
- DOI: 10.1016/j.mtbio.2025.101477
Hydrogel inspired by "adobe" with antibacterial and antioxidant properties for diabetic wound healing
Abstract
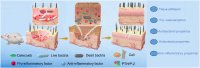
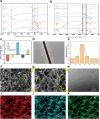
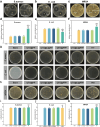
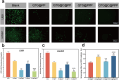
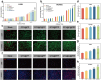
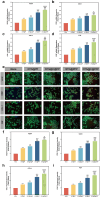
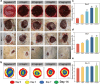
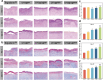
With the aging population, the incidence of diabetes is increasing. Diabetes often leads to restricted neovascularization, antibiotic-resistant bacterial infections, reduced wound perfusion, and elevated reactive oxygen species, resulting in impaired microenvironments and prolonged wound healing. Hydrogels are important tissue engineering materials for wound healing, known for their high water content and good biocompatibility. However, most hydrogels suffer from poor mechanical properties and difficulty in achieving sustained drug release, hindering their clinical application. Inspired by the incorporation of fibers to enhance the mechanical properties of "adobe," core-shell fibers were introduced into the hydrogel. This not only improves the mechanical strength of the hydrogel but also enables the possibility of sustained drug release. In this study, we first prepared core-shell fibers with PLGA (poly(lactic-co-glycolic acid)) and PCL (polycaprolactone). PLGA was loaded with P2 (Parathyroid hormone-related peptides-2), developed by our group, which promotes angiogenesis and cell proliferation. We then designed a QTG (QCS/TA/Gel, quaternary ammonium chitosan/tannic acid/gelatin) hydrogel, incorporating the core-shell fibers and the anti-inflammatory drug celecoxib into the QTG hydrogel. This hydrogel exhibits excellent antibacterial properties and biocompatibility, along with good mechanical performance. This hydrogel demonstrates excellent water absorption and swelling capabilities. In the early stages of wound healing, the hydrogel can absorb the wound exudate, maintaining the stability of the wound microenvironment. This hydrogel promotes neovascularization and collagen deposition, accelerating the healing of diabetic wounds, with a healing rate exceeding 95 % by day 14. Overall, this study provides a promising strategy for developing tissue engineering scaffolds for diabetic wound healing.
Keywords: Antibacterial; Antioxidant; Core-shell fibers; Diabetic wound healing; Hydrogel.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Nanofiber/hydrogel core-shell scaffolds with three-dimensional multilayer patterned structure for accelerating diabetic wound healing.J Nanobiotechnology. 2022 Jan 8;20(1):28. doi: 10.1186/s12951-021-01208-5. J Nanobiotechnology. 2022. PMID: 34998407 Free PMC article.
-
Injectable Nanocomposite Hydrogel for Accelerating Diabetic Wound Healing Through Inflammatory Microenvironment Regulation.Int J Nanomedicine. 2025 Feb 6;20:1679-1696. doi: 10.2147/IJN.S505918. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39931526 Free PMC article.
-
Tissue-Adhesive and Antibacterial Hydrogel Promotes MDR Bacteria-Infected Diabetic Wound Healing via Disrupting Bacterial Biofilm, Scavenging ROS and Promoting Angiogenesis.Adv Healthc Mater. 2025 Apr;14(10):e2404889. doi: 10.1002/adhm.202404889. Epub 2025 Feb 11. Adv Healthc Mater. 2025. PMID: 39935129
-
Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing.Acta Biomater. 2022 Jul 1;146:119-130. doi: 10.1016/j.actbio.2022.04.041. Epub 2022 Apr 26. Acta Biomater. 2022. PMID: 35483628
-
Advancing diabetic wound care: The role of copper-containing hydrogels.Heliyon. 2024 Sep 26;10(20):e38481. doi: 10.1016/j.heliyon.2024.e38481. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39640763 Free PMC article. Review.
Cited by
-
FGF mimetic peptide-modified electrospun nanocomposite fibrous membranes for accelerating infectious diabetic wound healing by synergistic antibacterial and pro-angiogenesis effects.Mater Today Bio. 2025 May 17;32:101877. doi: 10.1016/j.mtbio.2025.101877. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40496728 Free PMC article.
References
-
- Chang M., Nguyen T.T. Strategy for treatment of infected diabetic foot ulcers. Acc. Chem. Res. 2021;54(5):1080–1093. - PubMed
-
- Castleberry S.A., Almquist B.D., Li W., Reis T., Chow J., Mayner S., et al. Self-assembled wound dressings silence MMP-9 and improve diabetic wound healing in vivo. Adv Mater. 2016;28(9):1809–1817. - PubMed
LinkOut - more resources
Full Text Sources

