Health-related quality of life outcomes reporting associated with FDA approvals in haematology and oncology
- PMID: 39886148
- PMCID: PMC11256025
- DOI: 10.1136/bmjonc-2024-000369
Health-related quality of life outcomes reporting associated with FDA approvals in haematology and oncology
Abstract
Objective: Health-related quality of life (HRQoL) outcomes are important in making clinical and policy decisions. This study aimed to examine the HRQoL reporting in cancer drug trials leading to Food and Drug Administration (FDA) approvals.
Methods and analysis: This retrospective cohort study analysed HRQoL data for trials leading to FDA approvals between July 2015 and May 2020. Proportion of included trials that reported HRQoL, latency between FDA approval and first report of HRQoL data, HRQoL outcomes, and their correlation with OS (overall survival) and PFS (progression-free survival) were analysed.
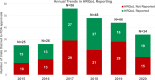
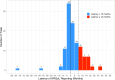
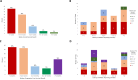
Results: Of the 233 trials associated with 207 FDA approvals, HRQoL was reported in 50% of trials, of which only 42% had the data reported by the time of FDA approval. There were no changes in frequency of HRQoL reporting between 2015 and 2020. HRQoL data were first reported in the primary publication in only 30% trials. Of the 115 trials with HRQoL data available, HRQoL improved in 43%, remained stable in 53% and worsened in 4% of trials. Among the trials that led to FDA approvals based on surrogate endpoints (79%), HRQoL was reported in 45% and improved only in 18% trials. There was no association between OS and PFS benefit and HRQoL outcomes.
Conclusion: Rates of HRQoL reporting were suboptimal in trials that led to FDA approvals with no improvements seen between 2015 and 2020. HRQoL reporting was often delayed and not presented in the primary publication. HRQoL reporting was further sparse in trials with approvals based on surrogate endpoints and HRQoL improved in only a minority of them.
Keywords: Medical oncology; Palliative care.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Estimation of Study Time Reduction Using Surrogate End Points Rather Than Overall Survival in Oncology Clinical Trials.JAMA Intern Med. 2019 May 1;179(5):642-647. doi: 10.1001/jamainternmed.2018.8351. JAMA Intern Med. 2019. PMID: 30933235 Free PMC article.
-
Food and Drug Administration approvals in phase 3 Cancer clinical trials.BMC Cancer. 2021 Jun 12;21(1):695. doi: 10.1186/s12885-021-08457-5. BMC Cancer. 2021. PMID: 34118915 Free PMC article. Clinical Trial.
-
Analysis of Control Arm Quality in Randomized Clinical Trials Leading to Anticancer Drug Approval by the US Food and Drug Administration.JAMA Oncol. 2019 Jun 1;5(6):887-892. doi: 10.1001/jamaoncol.2019.0167. JAMA Oncol. 2019. PMID: 31046071 Free PMC article.
-
Trends in endpoint use in pivotal trials and efficacy for US Food and Drug Administration-approved solid tumor therapies, 1995-2021.J Manag Care Spec Pharm. 2022 Nov;28(11):1219-1223. doi: 10.18553/jmcp.2022.28.11.1219. J Manag Care Spec Pharm. 2022. PMID: 36282934 Free PMC article. Review.
-
Assessment of the Clinical Benefit of Cancer Drugs Receiving Accelerated Approval.JAMA Intern Med. 2019 Jul 1;179(7):906-913. doi: 10.1001/jamainternmed.2019.0462. JAMA Intern Med. 2019. PMID: 31135808 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
