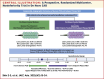
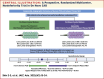
A Prospective Randomized Trial Comparing 2 Different Paclitaxel-Coated Balloons in De Novo Coronary Artery Disease
- PMID: 39886190
- PMCID: PMC11775799
- DOI: 10.1016/j.jacasi.2024.10.028
A Prospective Randomized Trial Comparing 2 Different Paclitaxel-Coated Balloons in De Novo Coronary Artery Disease
Abstract
Background: The Genoss paclitaxel-coated balloon (PCB) is a novel PCB with shellac and vitamin E as excipients, enhancing drug delivery to the target lesion and minimizing restenosis.
Objectives: This study aimed to compare quantitative coronary angiographic outcomes at 6 months after treatment of de novo coronary artery disease (CAD) with 2 different types of PCBs.
Methods: This prospective, multicenter, noninferiority trial randomized 204 patients with chronic coronary syndrome or stabilized acute coronary syndrome to treatment with the shellac and vitamin E-based PCB or the reference PCB (SeQuent Please NEO) in a 1:1 ratio. The primary endpoint was noninferiority for the 6-month angiographic in-lesion late lumen loss.
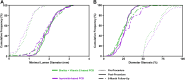
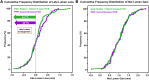
Results: The 6-month in-lesion late lumen loss was 0.06 ± 0.38 mm with shellac and vitamin E-based PCB vs 0.09 ± 0.36 mm with reference PCB. The 1-sided 97.5% upper confidence limit of the difference was 0.08 mm, which was lower than the noninferiority limit of 0.15 mm, achieving noninferiority (P for noninferiority = 0.001). There was comparable late lumen enlargement (44.7% vs 42.7%; P = 0.903) and binary restenosis rates (3.2% vs 6.7%; P = 0.442) following treatment with shellac and vitamin E-based PCB and reference PCB, respectively. Both PCBs had similar 12-month rates of target vessel failure (3.0% in shellac and vitamin E-based PCB vs 4.3% in reference PCB; P = 0.921).
Conclusions: The Genoss PCB, formulated with shellac and vitamin E as excipients, demonstrated angiographic outcomes comparable to a clinically proven PCB in the treatment of de novo CAD. (Compare the Safety and Efficacy of Genoss® DCB and SeQuent® Please NEO in Coronary De Novo Lesions; NCT05096442).
Keywords: drug-coated balloon; outcome; paclitaxel; percutaneous coronary intervention; randomized controlled trial.
© 2025 The Authors.
Conflict of interest statement
This study was supported by the unrestricted research fund from GENOSS Co Ltd (Suwon, South Korea) through an institutional research grant. The executive committee, together with the sponsor, designed the clinical trial. The sponsor had no role in data collection, data analysis, data interpretation, writing the manuscript, or the decision to submit the manuscript for publication. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. - PubMed
-
- Jeger R.V., Farah A., Ohlow M.A., et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. 2018;392:849–856. - PubMed
-
- Jeger R.V., Farah A., Ohlow M.A., et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. 2020;396:1504–1510. - PubMed
-
- Rissanen T.T., Uskela S., Eränen J., et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): a single-blind, randomised, non-inferiority trial. Lancet. 2019;394:230–239. - PubMed
-
- Shin E.S., Jun E.J., Kim S., et al. Clinical impact of drug-coated balloon-based percutaneous coronary intervention in patients with multivessel coronary artery disease. JACC Cardiovasc Interv. 2023;16:292–299. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

