P2X7R antagonism suppresses long-lasting brain hyperexcitability following traumatic brain injury in mice
- PMID: 39886340
- PMCID: PMC11780721
- DOI: 10.7150/thno.97254
P2X7R antagonism suppresses long-lasting brain hyperexcitability following traumatic brain injury in mice
Abstract
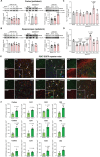
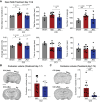
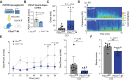
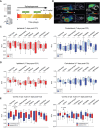
Purpose: Post-traumatic epilepsy (PTE) is one of the most common life-quality reducing consequences of traumatic brain injury (TBI). However, to date there are no pharmacological approaches to predict or to prevent the development of PTE. The P2X7 receptor (P2X7R) is a cationic ATP-dependent membrane channel that is expressed throughout the brain. While increasing evidence suggests a role for the P2X7R during seizures and epilepsy, it is unclear if changes in P2X7R expression can predict TBI-induced epilepsy development, and whether P2X7R antagonism can protect against long-lasting brain hyperexcitability caused by TBI. Methods: TBI was induced in adult male mice using the controlled cortical impact model (CCI). To test the anti-epileptogenic effects of P2X7R antagonism, mice were treated with brain-penetrant P2X7R antagonists JNJ-54175446 (30 mg/kg) or AFC-5128 (30 mg/kg) for 7 days post-CCI. The cell-type specific effects of P2X7Rs on TBI-induced hyperexcitability were analyzed in mice lacking exon 2 of the P2rx7 gene selectively in microglia (P2rx7:Cx3cr1-Cre). Static positron emission tomography (PET) via an intravenous injection of the P2X7R radioligand 18F-JNJ-64413739 and magnetic resonance imaging (MRI) were conducted twice during the first- and third-week post-injury. Results: Following TBI, while there were no obvious changes in P2X7R protein levels in the ipsilateral hippocampus post-injury, there was a delayed increase in P2X7R protein levels in the ipsilateral cortex at 3 months post-injury. Treatment with P2X7R antagonists shortly after TBI reduced long-lasting brain hyperexcitability, reduced cortical contusion volume, and normalized injury-induced hyperactivity to control sham-levels at 3 weeks post-TBI. Notably, mice lacking P2rx7 in microglia had an increased seizure threshold after TBI, suggesting that P2X7R contributed to brain hyperexcitability via its effects on microglia. Finally, P2X7R radioligand uptake after TBI correlated with seizure threshold at 3 weeks post-injury. Conclusions: Our results demonstrate the antiepileptogenic potential of P2X7R antagonism to prevent TBI-induced epilepsy and indicate that P2X7R-based PET imaging may be a useful diagnostic tool to identify people at risk of developing PTE.
Keywords: Epileptogenesis; Magnetic Resonance Imaging; Microglia; P2X7 Receptor; Positron Emission Tomography; Post-traumatic Epilepsy; Traumatic Brain Injury.
© The author(s).
Conflict of interest statement
Competing interests: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare that this study received funding from FutureNeuro. Bert Klebl, Klaus Dinkel and Michael Hamacher are stakeholders in KHAN Technology, Affectis Pharmaceuticals and Lead Discovery Center GmbH respectively but had no influence in the study design. Anindya Bhattacharya, James Palmer, and Marc Ceusters are/were employees of Janssen Research and Development.
Figures



References
-
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M. et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130:1080–97. - PubMed
-
- Agrawal A, Timothy J, Pandit L, Manju M. Post-traumatic epilepsy: an overview. Clin Neurol Neurosurg. 2006;108:433–9. - PubMed
-
- Ferguson PL, Smith GM, Wannamaker BB, Thurman DJ, Pickelsimer EE, Selassie AW. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia. 2010;51:891–8. - PubMed
-
- Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

