Merging Copper Catalysis with Nitro Allyl and Allyl Sulfone Derivatives: Practical, Straightforward, and Scalable Synthesis of Diversely Functionalized Allyl Boranes
- PMID: 39886564
- PMCID: PMC11775705
- DOI: 10.1021/jacsau.4c00809
Merging Copper Catalysis with Nitro Allyl and Allyl Sulfone Derivatives: Practical, Straightforward, and Scalable Synthesis of Diversely Functionalized Allyl Boranes
Abstract
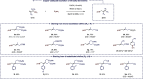
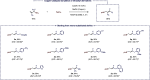
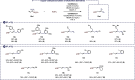
We report here the first example of a copper-catalyzed transformation involving nitro allyl derivatives. This borylation reaction, which exploits the high versatility of the aforementioned precursor, tolerates a variety of functional groups and allows practical, scalable, and highly straightforward access to diversely substituted allylboronic esters in high yields. The method was also extended to allyl sulfones, which provides a very complementary approach, offering additional structural diversity along with improved stereoselectivities. This new reactivity was further exploited to synthesize γ-fluoroallyl boronic esters as well as various synthetically useful building blocks through key post-functionalizations. Both the reaction mechanism and the chemoselectivity were rationalized experimentally and through DFT calculations.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Nickel-Catalyzed Highly Selective Hydroalkenylation of Alkenyl Boronic Esters to Access Allyl Boron.Org Lett. 2020 Nov 6;22(21):8285-8290. doi: 10.1021/acs.orglett.0c02923. Epub 2020 Oct 22. Org Lett. 2020. PMID: 33089688
-
Copper-catalyzed enantioselective allyl-allyl cross-coupling.J Am Chem Soc. 2013 Feb 13;135(6):2140-3. doi: 10.1021/ja312487r. Epub 2013 Feb 1. J Am Chem Soc. 2013. PMID: 23350620
-
Organophotocatalyzed Alkyl/Arylsulfonylation of Vinylcyclopropanes.Chemistry. 2024 Jan 22;30(5):e202303187. doi: 10.1002/chem.202303187. Epub 2023 Dec 6. Chemistry. 2024. PMID: 37926681
-
Cobalt-catalyzed 1,4-hydrovinylation of allylsilane and allylboronic esters for the synthesis of hydroxy-functionalized 1,4-dienes.J Org Chem. 2010 Aug 6;75(15):5203-10. doi: 10.1021/jo100951d. J Org Chem. 2010. PMID: 20597480
-
Beyond classical sulfone chemistry: metal- and photocatalytic approaches for C-S bond functionalization of sulfones.Chem Soc Rev. 2022 Aug 1;51(15):6774-6823. doi: 10.1039/d0cs00535e. Chem Soc Rev. 2022. PMID: 35838659 Review.
References
-
- Wuts P. G. M.; Bigelow S. S. Application of allylboronates to the synthesis of carbomycin B. J. Org. Chem. 1988, 53, 5023–5034. 10.1021/jo00256a023. - DOI
-
- Roush W. R.Applications of allylboronates in the synthesis of carbohydrates and polyhydroxylated natural products in Trends in Synthetic Carbohydrate Chemistry. ACS Symposium Series American Chemical Society, 1989; Vol. 386, pp 242–277.
-
- Kabalka G. W.; Venkataiah B. The total synthesis of eupomatilones 2 and 5. Tetrahedron Lett. 2005, 46, 7325–7328. 10.1016/j.tetlet.2005.08.140. - DOI
LinkOut - more resources
Full Text Sources
