H2O2 Triggering Electron-Directed Transfer of Emerging Contaminants over Asymmetric Nano Zinc Oxide Surfaces for Water Self-Purification Expansion
- PMID: 39886598
- PMCID: PMC11775690
- DOI: 10.1021/jacsau.4c00950
H2O2 Triggering Electron-Directed Transfer of Emerging Contaminants over Asymmetric Nano Zinc Oxide Surfaces for Water Self-Purification Expansion
Abstract
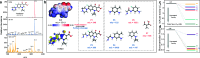
Slow mass transfer processes between inert emerging contaminants (ECs) and dissolved oxygen (DO) limit natural water self-purification; thus, excessive energy consumption is necessary to achieve ECs removal, which has become a longstanding global challenge. Here, we propose an innovative water self-purification expansion strategy by constructing asymmetric surfaces that could modulate trace H2O2 as trigger rather than oxidant to bridge a channel between inert ECs and natural dissolved oxygen, achieved through a dual-reaction-center (DRC) catalyst consisting of Cu/Co lattice-substituted ZnO nanorods in situ (CCZO-NRs). During water purification, the bond lengths of emerging contaminants (ECs) adsorbed on the asymmetric surface were stretched, and this stretching was further enhanced by H2O2 mediation, resulting in a significant reduction of bond-breaking energy barriers. As a result, the consumption rate of H2O2 was reduced by two-thirds in the presence of ECs. In contrast, the removal of ECs was increased approximately 95-fold mediated by trace H2O2. It exhibits the highest catalytic performance with the lowest dosage of H2O2 among numerous similarly reported systems. This discovery is significant for the development of water self-purification expansion technologies.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Endogenous Substances Utilization for Water Self-Purification Amplification Driven by Nonexpendable H2O2 over a Micro-Potential Difference Surface.Environ Sci Technol. 2024 Dec 31;58(52):23241-23250. doi: 10.1021/acs.est.4c09385. Epub 2024 Dec 16. Environ Sci Technol. 2024. PMID: 39680062
-
Simultaneous Emerging Contaminant Removal and H2O2 Generation Through Electron Transfer Carrier Effect of Bi─O─Ce Bond Bridge Without External Energy Consumption.Adv Sci (Weinh). 2024 Aug;11(29):e2308519. doi: 10.1002/advs.202308519. Epub 2024 Jun 3. Adv Sci (Weinh). 2024. PMID: 38831633 Free PMC article.
-
Water Self-Purification with Zero External Consumption by Livestock Manure Resource Utilization.Environ Sci Technol. 2023 Feb 21;57(7):2837-2845. doi: 10.1021/acs.est.2c09163. Epub 2023 Feb 11. Environ Sci Technol. 2023. PMID: 36773285
-
Selected emerging contaminants in water: Global occurrence, existing treatment technologies, regulations and associated risk.J Hazard Mater. 2025 Feb 5;483:136541. doi: 10.1016/j.jhazmat.2024.136541. Epub 2024 Nov 20. J Hazard Mater. 2025. PMID: 39608075 Review.
-
Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes - A review.Chemosphere. 2017 May;174:665-688. doi: 10.1016/j.chemosphere.2017.02.019. Epub 2017 Feb 4. Chemosphere. 2017. PMID: 28199944 Review.
References
-
- Hou Y.; Liu F.; Zhang B.; Tong M. Thiadiazole-Based Covalent Organic Frameworks with a Donor–Acceptor Structure: Modulating Intermolecular Charge Transfer for Efficient Photocatalytic Degradation of Typical Emerging Contaminants. Environ. Sci. Technol. 2022, 56 (22), 16303–16314. 10.1021/acs.est.2c06056. - DOI - PubMed
-
- Gomes I. B.; Maillard J.-Y.; Simões L. C.; Simões M. Emerging contaminants affect the microbiome of water systems—strategies for their mitigation. npj Clean Water 2020, 3 (1), 39.10.1038/s41545-020-00086-y. - DOI
-
- Chen J.; Qin C.; Mou Y.; Cao Y.; Chen H.; Yuan X.; Wang H. Linker regulation of iron-based MOFs for highly effective Fenton-like degradation of refractory organic contaminants. Chem. Eng. J. 2023, 459, 14158810.1016/j.cej.2023.141588. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
