Transcriptomics and proteomics characterizing the antioxidant mechanisms of semaglutide in diabetic mice with cognitive impairment
- PMID: 39886945
- PMCID: PMC11819768
- DOI: 10.3892/ijmm.2025.5497
Transcriptomics and proteomics characterizing the antioxidant mechanisms of semaglutide in diabetic mice with cognitive impairment
Abstract
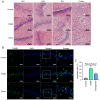
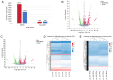
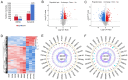
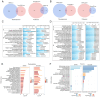
The aim of the present study was to investigate the neuroprotective effects of semaglutide in diabetes‑associated cognitive decline (DACD), while also exploring the underlying mechanisms targeting anti‑oxidative effects. The present study evaluated the antioxidant properties of semaglutide using a DACD model of inflammation. To investigate the underlying mechanisms, omics technologies were employed. Comprehensive transcriptomic and proteomic analysis of the cells was conducted to identify the pathways responsible for the observed antioxidant effects. Semaglutide demonstrated the potential to enhance learning and memory functions while mitigating hippocampal pathological damage. RNA‑sequencing and data‑independent acquisition proteomics analyses identified 13,511 differentially expressed genes and 588 differentially expressed proteins between the control and type 2 diabetes mellitus (T2DM) groups. In addition, 1,378 genes and 2,394 proteins exhibited a differential expression between the T2DM and semaglutide (10 µg/kg) treatment groups. A combined transcriptomic and proteomic analysis unveiled 40 common pathways. Acyl‑CoA oxidase 1 (ACOX1) was observed to be activated during oxidative stress and subsequently suppressed by semaglutide. Of note, the antioxidant and anti‑apoptotic properties of semaglutide in high glucose (HG) conditions were partially reversed upon ACOX1 overexpression. Overall, the present data provided molecular evidence to elucidate the physiological connections between semaglutide and neuronal function, and contribute to clarifying the role of semaglutide in combating oxidative stress and HG‑induced cognitive impairment.
Keywords: cognitive; diabetes; oxidative stress; proteomic; transcriptomic.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
