Triptolide reverses cis‑diamminedichloroplatinum resistance in esophageal squamous cell carcinoma by suppressing glycolysis and causing mitochondrial malfunction
- PMID: 39886972
- PMCID: PMC11795233
- DOI: 10.3892/mmr.2025.13439
Triptolide reverses cis‑diamminedichloroplatinum resistance in esophageal squamous cell carcinoma by suppressing glycolysis and causing mitochondrial malfunction
Abstract
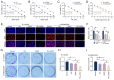
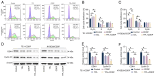
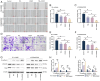
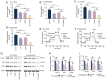
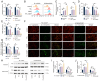
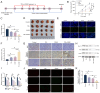
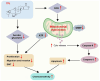
The present study investigated the sensitization mechanism of triptolide (TPL) in esophageal squamous cell carcinoma (ESCC) resistant to cis‑diamminedichloroplatinum (CDDP). CDDP‑resistant TE‑1/CDDP and KYSE30/CDDP cells were created using an incremental drug concentration approach. TPL and CDDP treatment conditions were screened based on the Cell Counting Kit‑8 cell viability assay and cell proliferation was detected using 5‑ethynyl‑2'‑deoxyuridine and clone formation assays. Flow cytometry combined with Hoechst 33258 staining was used to assess cell cycle progression and apoptosis. Scratch healing assay, Transwell assay and western blotting were used to investigate the malignant behaviors of the cells. Changes in cellular glycolysis were investigated by measuring glucose uptake, lactate production and the levels of related regulatory factors. Changes in mitochondrial function were examined by detecting ATP and reactive oxygen species levels, as well as mitochondrial membrane potential and cytochrome c release. Furthermore, a nude mouse subcutaneous graft tumor model assay was used to assess the in vivo effect of TPL. In vitro dosages of TPL and CDDP were tested at 2 nM and 4 µM, respectively. Notably, TPL decreased the proliferation, migration, invasion and epithelial‑mesenchymal transition of CDDP‑resistant ESCC cells, increased their apoptosis and significantly suppressed tumor growth in a nude mouse model of ESCC. TPL was shown to have a strong CDDP‑sensitizing effect in vitro and in vivo and its mechanism may involve inhibiting anaerobic glycolysis and causing mitochondrial energy metabolism impairment to induce apoptosis. In conclusion, TPL may be considered a potential CDDP sensitizer with substantial clinical implications for ESCC therapy.
Keywords: cis‑diamminedichloroplatinum‑resistant; esophageal squamous cell carcinoma; glycolysis; mitochondrial dysfunction; triptolide.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Downregulation of ALDH5A1 suppresses cisplatin resistance in esophageal squamous cell carcinoma by regulating ferroptosis signaling pathways.Mol Carcinog. 2024 Oct;63(10):1892-1906. doi: 10.1002/mc.23778. Epub 2024 Jun 24. Mol Carcinog. 2024. PMID: 38923019
-
Luteolin enhances drug chemosensitivity by downregulating the FAK/PI3K/AKT pathway in paclitaxel‑resistant esophageal squamous cell carcinoma.Int J Mol Med. 2024 Sep;54(3):77. doi: 10.3892/ijmm.2024.5401. Epub 2024 Jul 12. Int J Mol Med. 2024. PMID: 38994756 Free PMC article.
-
α-Hederin Induces Apoptosis of Esophageal Squamous Cell Carcinoma via an Oxidative and Mitochondrial-Dependent Pathway.Dig Dis Sci. 2019 Dec;64(12):3528-3538. doi: 10.1007/s10620-019-05689-1. Epub 2019 Jul 4. Dig Dis Sci. 2019. PMID: 31273592
-
IFI6 depletion inhibits esophageal squamous cell carcinoma progression through reactive oxygen species accumulation via mitochondrial dysfunction and endoplasmic reticulum stress.J Exp Clin Cancer Res. 2020 Jul 29;39(1):144. doi: 10.1186/s13046-020-01646-3. J Exp Clin Cancer Res. 2020. PMID: 32727517 Free PMC article.
-
Periplcymarin targets glycolysis and mitochondrial oxidative phosphorylation of esophageal squamous cell carcinoma: Implication in anti-cancer therapy.Phytomedicine. 2024 Jun;128:155539. doi: 10.1016/j.phymed.2024.155539. Epub 2024 Mar 15. Phytomedicine. 2024. PMID: 38522311
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

