Supporting endocrine therapy adherence in women with breast cancer: findings from the ROSETA pilot fractional factorial randomized trial
- PMID: 39887069
- PMCID: PMC11783298
- DOI: 10.1093/abm/kaaf003
Supporting endocrine therapy adherence in women with breast cancer: findings from the ROSETA pilot fractional factorial randomized trial
Abstract
Background: Adherence to adjuvant endocrine therapy (AET) in women with breast cancer is poor. Multicomponent intervention packages are needed to address adherence barriers. Optimizing these packages prior to definitive evaluation can increase their effectiveness, affordability, scalability, and efficiency.
Purpose: To pilot procedures for an optimization-randomized controlled trial (O-RCT) of the 'Refining and Optimizing Strategies to support Endocrine Therapy Adherence' (ROSETA) intervention.
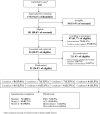
Methods: This was a multisite individually randomized external pilot trial using a 24-1 fractional factorial design (ISRCTN10487576). Breast cancer survivors prescribed AET were recruited from 5 hospitals and randomized to one of 8 conditions, each comprising a combination of 4 intervention components set to "on" or "off" (SMS messages, information leaflet, guided self-help, and self-management website). We set criteria to inform the decision to progress to an O-RCT for consent rate, component adherence, and availability of outcome measures, with predefined cutoffs for "green" (proceed), "amber" (minor changes), and "red" (major changes).
Results: Among 141 eligible patients, 54 (38.3%) consented (green range). At least 50.0% of participants adhered to the minimum threshold set for each intervention component (green range). Data for one of the 3 medication adherence measures were available (amber range). Most (86.8%) participants were satisfied with their trial experience. Exploratory analysis indicated some evidence of a negative main effect of the information leaflet on medication adherence (adjusted mean difference = 0.088, 95% CI, 0.018, 0.158).
Conclusions: Progression to a fully powered O-RCT of the ROSETA intervention package is feasible, but review of medication adherence measures is required.
Keywords: acceptance and commitment therapy; breast cancer; factorial trial; medication adherence; optimization; text messaging.
Plain language summary
Most women with breast cancer are prescribed a medication called adjuvant endocrine therapy (AET) to reduce the chance of breast cancer returning. However, many women struggle to take (adhere to) AET for several reasons. We developed an intervention with 4 components to target key barriers to taking AET: text messages, information leaflet, guided self-help, and side effect self-management website. We conducted a small trial to answer key questions ahead of a larger trial. Women prescribed AET were recruited from 5 hospitals in the United Kingdom. They were randomly allocated to one of 8 groups made up of different combinations of the 4 intervention components. We set out criteria to help us decide whether to proceed to a larger trial. One hundred forty-one women were eligible to take part, of which 54 (38.3%) agreed to participate. At least half of the participants engaged with the minimum level of each intervention component. We could only get data for one of our 3 assessments of AET adherence. Most (86.8%) participants were satisfied with their trial experience. Overall, we met the criteria we set to proceed to a larger trial, but we will need to review how we measure medication adherence.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Society of Behavioral Medicine.
Conflict of interest statement
S.G.S. declares consulting fees from Lily for participation in an advisory board. R.F. chairs the Implementation Strategy Group of the UK National Institute for Health and Care Excellence. M.C. declares being a Data Monitoring and Ethics Committee and Trial Steering Committee member of NIHR funded projects and being a member of NIHR grant funding panels. G.V. declares receiving honoraria from Pfizer, Novartis, Eisai, and Lilly, consultancy fees for advisory board membership from AstraZeneca, Roche, Novartis, Pfizer, Seagen, Eisai, Sanofi, and receipt of an institutional grant from Pfizer. All other authors declare no conflict of interest.
References
-
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15-23. https://doi.org/ 10.1016/j.breast.2022.08.010 - DOI - PMC - PubMed
-
- Lin L, Yan L, Liu Y, Yuan F, Li H, Ni J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J Hematol Oncol. 2019;12:96. https://doi.org/ 10.1186/s13045-019-0783-9 - DOI - PMC - PubMed
-
- Shelton J, Zotow E, Smith L, et al. 25 year trends in cancer incidence and mortality among adults aged 35-69 years in the UK, 1993-2018: retrospective secondary analysis. BMJ. 2024;384:e076962. https://doi.org/ 10.1136/bmj-2023-076962 - DOI - PMC - PubMed
-
- Cancer Research UK. Breast cancer statistics. 2021. Accessed January 20, 2025. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s...
-
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451. https://doi.org/ 10.3322/caac.21583 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- National Institute for Health Research
- NIHR300588/NIHR Advanced Fellowship
- L389SS/Yorkshire Cancer Research University Academic Fellowship
- IS-BRC-1215-20007/NIHR Manchester Biomedical Research Centre
- NIHR134141/NIHR Health Technology Assessment
- RP-PG-1016-20005/NIHR Programme Grants for Applied Research
- NIHR151848/NIHR Health and Social Care Delivery Research
- NIHR204293/NIHR Patient Safety Research Collaboration
- NIHR200166/NIHR Applied Research Collaboration
- NIHR203334/NIHR Blood Transfusion Research Unit
- RP-PG-0216-20003/NIHR Programme Grants for Applied Research
- NIHR301709/NIHR Advanced Fellowship
- 6488035/Macmillan Cancer Support
- L417/YCR_/Yorkshire Cancer Research/United Kingdom
- 2019DecPR1367/BBC_/Breast Cancer Now/United Kingdom
- APP11/1/British Lung Foundation
- PB-PG-0816-20015/NIHR Research for Patient Benefit
- RP-PG-1016-20007/NIHR Programme Grants for Applied Research
- NIHR135081/NIHR Public Health Research
- 15/43/07/NIHR Health Technology Assessment
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous