A more rapid method for transformation of Helicobacter pylori
- PMID: 39887209
- PMCID: PMC11853076
- DOI: 10.1128/msphere.00005-25
A more rapid method for transformation of Helicobacter pylori
Abstract
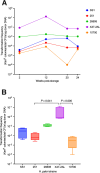
Helicobacter pylori is a major causative agent in several upper gastroduodenal tract diseases, including gastric cancer. The development of methods to genetically manipulate H. pylori by natural transformation has allowed a greater understanding of its biology and role in these diseases. Nevertheless, the transformation methods used for H. pylori are time-consuming, requiring growth of these fastidious and slow-growing bacteria from -80°C stocks. The aim of the study was to develop a more rapid and convenient method for generating H. pylori mutants. We describe here a method in which competent H. pylori bacteria can be stored at -80°C and used in transformations on the day of resuscitation, similar to methods routinely used for Escherichia coli. This means that transformation can be performed at will and that transformants can be obtained within days, rather than weeks. Furthermore, we show that bacteria remain competent for at least six months storage at -80°C and that the method is applicable to strains with varying levels of natural competence. Transformation efficiencies of the bacteria varied between 101 and 106 transformants/total colony-forming units/µg donor DNA, depending on the strain. We suggest that this improved method will facilitate studies on H. pylori and, moreover, may be applicable to other naturally transformable pathogens with fastidious growth requirements and requiring ultra-low temperature refrigeration for long-term preservation.IMPORTANCEGenetic manipulation is an important tool in the study of pathogenic bacteria and their interactions with the host. Many pathogenic bacteria are naturally transformable; however, transformation experiments can be impeded by the slow-growing and fastidious nature of some species. One such bacterium is Helicobacter pylori, which requires resuscitation from -80°C and multiple subcultures prior to transformation. The method described in the current study uses a simple modification of a conventional method of natural transformation. Using this method, competent H. pylori bacteria can be stored for long periods (at least six months) and resuscitated as needed for use in experiments. The method circumvents the need for multiple and lengthy subcultures prior to transformation, nor does it involve costly materials, complicated procedures, or sophisticated equipment. Thus, we describe a simple, inexpensive, and time-efficient method that may have broader applications for use with other fastidious bacteria.
Keywords: Helicobacter pylori; mutagenesis; naturally competent; pathogen; transformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
