Biomarker treatment effects in two phase 3 trials of gantenerumab
- PMID: 39887500
- PMCID: PMC11848197
- DOI: 10.1002/alz.14414
Biomarker treatment effects in two phase 3 trials of gantenerumab
Abstract
Introduction: We report biomarker treatment effects in the GRADUATE I and II phase 3 studies of gantenerumab in early Alzheimer's disease (AD).
Methods: Amyloid and tau positron emission tomography (PET), volumetric magnetic resonance imaging (vMRI), cerebrospinal fluid (CSF), and plasma biomarkers used to assess gantenerumab treatment related changes on neuropathology, neurodegeneration, and neuroinflammation over 116 weeks.
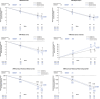
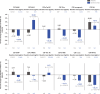
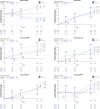
Results: Gantenerumab reduced amyloid PET load, CSF biomarkers of amyloid beta (Aβ)40, total tau (t-tau), phosphorylated tau 181 (p-tau181), neurogranin, S100 calcium-binding protein B (S100B), neurofilament light (NfL), alpha-synuclein (α-syn), neuronal pentraxin-2 (NPTX2), and plasma biomarkers of t-tau, p-tau181, p-tau217, and glial fibrillary acidic protein (GFAP) while increasing plasma Aβ40, Aβ42. vMRI showed increased reduction in whole brain volume and increased ventricular expansion, while hippocampal volume was unaffected. Tau PET showed no treatment effect.
Discussion: Robust treatment effects were observed for multiple biomarkers in GRADUATE I and II. Comparison across anti-amyloid antibodies indicates utility of p-tau and GFAP as biomarkers of amyloid plaque removal while NfL and tau PET seem unsuitable as consistent indicators of clinical efficacy. vMRI might be confounded by non-neurodegenerative brain volume changes. TRIAL REGISTRATION NUMBER (CLINICALTRIALS.GOV IDENTIFIER): NCT03444870 and NCT03443973.
Highlights: Gantenerumab significantly reduced brain amyloid load. Tau positron emission tomography showed no treatment effect in a small subset of participants. Volumetric magnetic resonance imaging showed increased whole brain volume reduction under treatment while hippocampal volume was unaffected. Robust treatment effects on cerebrospinal fluid and plasma biomarkers were found, despite lack of clinical efficacy.
Keywords: Alzheimer's disease; amyloid beta; anti‐amyloid; biomarkers; blood‐based biomarkers; cerebrospinal fluid; gantenerumab; neurofilament light; phase 3; phosphorylated tau 217; phosphorylated tau181; positron emission tomography; tau; volumetric magnetic resonance imaging.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Tobias Bittner is a full‐time employee of F. Hoffmann‐La Roche Ltd and Genentech, Inc., a member of the Roche Group, and owns stock in F. Hoffmann‐La Roche Ltd. Matteo Tonietto, Gregory Klein, Anton Belousov, Vittorio Illiano, Paul Delmar, and Susanna Gobbi are full‐time employees of F. Hoffmann‐La Roche Ltd and own stock in F. Hoffmann‐La Roche Ltd. At the time of the study, Christopher Galli was full‐time employee of F. Hoffmann‐La Roche Ltd and owned stock in F. Hoffmann‐La Roche Ltd. Marzia A. Scelsi is a full‐time employee of Roche Products Ltd. Nicola Voyle is a full‐time employee of Roche Products Ltd. and owns stock in F. Hoffmann‐La Roche Ltd. Muhamed Barakovic is an employee of Hays plc and a consultant for F. Hoffmann‐La Roche Ltd. Maryam Abaei, Erica Silvestri, and Antonio Napolitano are employees of A4P Consulting Ltd. and consultants for F. Hoffmann‐La Roche Ltd. Kaj Blennow has served as a consultant and on advisory boards for AC Immune, Acumen, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd., Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. Frederik Barkhof has served on steering committees or as data safety monitoring board member for Biogen, Merck, Eisai, and Prothena; as an advisory board member for Combinostics, Scottish Brain Sciences; and as a consultant for Roche, Celltrion, Rewind Therapeutics, Merck, and Bracco. He has research agreements with ADDI, Merck, Biogen, GE Healthcare, and Roche and is a co‐founder and shareholder of Queen Square Analytics Ltd. Author disclosures are available in the supporting information.
Figures



References
-
- Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197‐210. - PubMed
-
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9‐21. - PubMed
-
- Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti‐Abeta antibody demonstrates sustained cerebral amyloid‐beta binding and elicits cell‐mediated removal of human amyloid‐beta. J Alzheimers Dis. 2012;28(1):49‐69. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

