CAV1 Exacerbates Renal Tubular Epithelial Cell Senescence by Suppressing CaMKK2/AMPK-Mediated Autophagy
- PMID: 39887553
- PMCID: PMC12073896
- DOI: 10.1111/acel.14501
CAV1 Exacerbates Renal Tubular Epithelial Cell Senescence by Suppressing CaMKK2/AMPK-Mediated Autophagy
Abstract
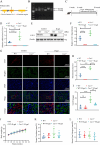
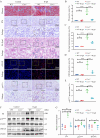
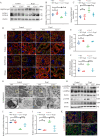
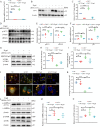
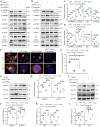
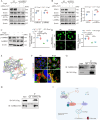
Renal proximal tubular epithelial cell (PTEC) senescence and defective autophagy contribute to kidney aging, but the mechanisms remain unclear. Caveolin-1 (CAV1), a crucial component of cell membrane caveolae, regulates autophagy and is associated with cellular senescence. However, its specific role in kidney aging is poorly understood. In this study, we generated Cav1 gene knockout mice and induced kidney aging using D-galactose (D-gal). The results showed that CAV1 expression increased in the renal cortex of the aging mice, which was accompanied by exacerbated renal interstitial fibrosis, elevated levels of senescence-associated proteins γH2AX and p16INK4a, and increased β-galactosidase activity. Moreover, autophagy and AMPK phosphorylation in PTECs were reduced. These phenotypes were partially reversed in D-gal-induced Cav1 knockout mice. Similar results were observed in D-gal-induced human proximal tubular epithelial (HK-2) cells, but these effects were blocked when AMPK activation was inhibited. Additionally, in CaMKK2 knockdown HK-2 cells, siCAV1 failed to promote AMPK phosphorylation, whereas this effect persisted when STK11 was knocked down. Besides, we examined the phosphorylation of CaMKK2 and found that siCAV1 increased its activity. Given that CaMKK2 activity is affected by intracellular Ca2+, we examined Ca2+ levels in HK-2 cells and found that D-gal treatment reduced intracellular Ca2+ concentration, but CAV1 knockdown did not alter these levels. Through GST pull-down assays, we demonstrated a direct interaction between CAV1 and CaMKK2. In conclusion, these findings suggest that CAV1 exacerbates renal tubular epithelial cell senescence by directly interacting with CaMKK2, suppressing its activity and AMPK-mediated autophagy via a Ca2+-independent pathway.
Keywords: AMPK; CAV1; CaMKK2; Autophagy; Kidney Aging; Renal Tubular Epithelial Cell.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Bolignano, D. , Mattace‐Raso F., Sijbrands E. J., and Zoccali C.. 2014. “The Aging Kidney Revisited: A Systematic Review.” Ageing Research Reviews 14: 65–80. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

