Multifunctional Fluorescent Probe for Simultaneous Detection of ATP, Cys, Hcy, and GSH: Advancing Insights into Epilepsy and Liver Injury
- PMID: 39887673
- PMCID: PMC11923924
- DOI: 10.1002/advs.202415882
Multifunctional Fluorescent Probe for Simultaneous Detection of ATP, Cys, Hcy, and GSH: Advancing Insights into Epilepsy and Liver Injury
Abstract
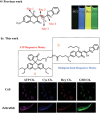
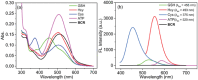
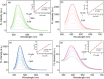
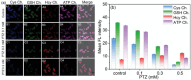
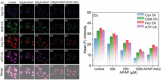
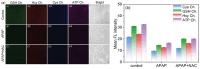
Adenosine triphosphate (ATP) is a critical intracellular energy currency that plays a key role in various cellular processes and is closely associated with numerous diseases. Similarly, biothiols such as glutathione (GSH), cysteine (Cys), and homocysteine (Hcy) are integral to many physiological and pathological processes due to their strong redox properties. Simultaneous discrimination and detection of ATP and biothiols offer valuable insights into the pathogenesis of conditions such as epilepsy and liver injury. This study introduces the first fluorescent probe, BCR, designed for multifunctional detection of ATP, GSH, Hcy, and Cys. With outstanding optical properties, excellent biocompatibility, high selectivity, and superior sensitivity, probe BCR enables effective imaging of ATP and biothiol dynamics in vivo. Moreover, probe BCR successfully visualizes changes in ATP, GSH, Hcy, and Cys levels in a PTZ-induced epileptic zebrafish model and an APAP-induced mouse liver injury tissue section model. These findings underscore the significant potential of probe BCR for early disease diagnosis and therapeutic applications.
Keywords: adenosine triphosphate; biothiols; fluorescent probe; simultaneous sensing.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chen H., Zhang Y.‐H. P. J., Crit. Rev. Biotechnol. 2021, 41, 1826403. - PubMed
-
- Jun Y. W., Wang T., Hwang S., Kim D., Ma D., Kim K. H., Kim S., Jung J., Ahn K. H., Angew. Chem., Int. Ed. 2018, 57, 10142. - PubMed
-
- Fang Y., Shi W., Hu Y., Li X., Ma H., Chem. Commun. 2018, 54, 5454. - PubMed
-
- Fortibui M. M., Yoon S. A., Yoo S. Y., Son J. Y., Lee M. H., Coord. Chem. Rev. 2024, 510, 215800.
-
- Seino S., Miki T., Prog. Biophys. Mol. Biol. 2003, 81, 133. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
