Inter-Laboratory Validation of Nodal/Paranodal Antibody Testing
- PMID: 39887819
- PMCID: PMC11780190
- DOI: 10.1111/jns.70000
Inter-Laboratory Validation of Nodal/Paranodal Antibody Testing
Abstract
Background and aims: Reliable detection of antibodies against nodal targets is vital for the diagnosis of autoimmune nodopathies. The performance characteristics of recently developed in-house assays are unknown. We compared testing at four centres.
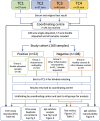
Methods: Each submitted 29-40 serum samples to a coordinating centre from one of three groups: (1) autoimmune nodopathy patients, with positive nodal/paranodal antibodies; (2) seronegative patients with other inflammatory neuropathies, and (3) healthy individuals or those with other neurological diseases. The coordinating centre recoded all samples and returned 160 identical aliquots to each testing centre for blinded testing. Once data from all centres had been received by the coordinating centre, unblinded results were returned for analysis. Sensitivity was defined by the proportion of group 1 samples returned as positive. Accuracy was defined as 0.075(sensitivity) + 0.925(specificity).
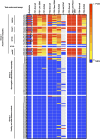
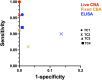
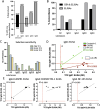
Results: Centres performed various combinations of ELISA, cell-based (CBAs) and teased-nerve fibre assays. All labs produced highly accurate results (96%-100%) and concordance for the overall result across at least 3 or all 4 test centres was observed for 98% and 89% of the samples respectively. However, 10/30 individual assays (6/14 CBAs and 4/16 ELISAs) were less than 90% sensitive. Only 3 assays had more than 1 false positive result (2 ELISAs and 1 CBA). Combining different assay modalities to produce an overall result did not improve accuracy. Inter-laboratory consistency in the determination of antibody subclasses was poor.
Interpretation: Although most samples were correctly categorised in all 4 centres, the use of a specific test modality or multiple tests did not guarantee accuracy. Early and repeated interlaboratory testing with sharing of samples is important to understand test performance and reproducibility, identify areas for improvement and maintain consistency. To aid this, we provide detailed methods for the best performing tests. Further standardisation of antibody subclass determination is required.
Keywords: CASPR1; autoantibodies; contactin‐1; immunoassay; neurofascin.
© 2025 The Author(s). Journal of the Peripheral Nervous System published by Wiley Periodicals LLC on behalf of Peripheral Nerve Society.
Conflict of interest statement
S.R. is a Medical Advisory Board member of the Guillain‐Barré syndrome and Associated Inflammatory Neuropathies (GAIN) patient charity. His department receives payment for the provision of diagnostic antibody testing, including of nodal/paranodal antibodies. He has received payment from Argenx for preparation and delivery of conference presentation on clinical trial, payment for serving on argenx, Annexon, Dianthus and Hansa clinical trial advisory boards, and payment from CSL for delivering a conference symposium lecture. Kathrin Doppler has received honoraria for lectures and presentations from Takeda, Grifols, Sanofi and Roche, and is a board member of the Inflammatory Neuropathy Consortium of the Peripheral Nerve Society. L.A. and K.D. have been supported by a scholarship from the Interdisciplinary Center of Clinical Research (IZKF) of the Faculty of Medicine, University of Würzburg (ZZ‐32, AdvCSP‐1) unrelated to the contents of this manuscript. R.H. is editorial board member of the Journal of the Peripheral Nervous System, a board member of the Inflammatory Neuropathy Consortium of the Peripheral Nerve Society, and reports funding from BÜHLMANN Laboratories AG outside the submitted work. Erasmus MC offers diagnostic services for testing of paranodal antibodies. M.T. was supported by the Erasmus MC Pain Foundation, has received funding from ZonMw (Memorabel programme), the Dutch EpilepsieNL Foundation (NEF 19‐08), Dioraphte (2001 0403) and E‐RARE JTC 2018 (UltraAIE, 90030376505). M.T. has filed a patent, on behalf of the Erasmus MC, for methods for typing neurological disorders and cancer, and devices for use therein, and has received research funds for serving on a scientific advisory board of Horizon Therapeutics/AmGen and ArgenX, for consultation at Guidepoint Global LLC, for consultation at UCB. MT has received an unrestricted research grant from CSL Behring. M.T. has received royalties from UpToDate Inc. L.Q. is supported by INT23/00066 and PI22/00387 from Instituto de Salud Carlos III—Ministry of Economy and Innovation (Spain) and received research grants from CIBERER, Fundació La Marató, GBS‐CIDP Foundation International, UCB and ArgenX. L.Q. received speaker or expert testimony honoraria from CSL Behring, Novartis, Sanofi‐Genzyme, Merck, Annexon, Alnylam, Janssen, ArgenX, UCB, Dianthus, LFB, Avilar Therapeutics, Lycia Therapeutics, Nuvig Therapeutics, Takeda and Roche. L.Q. serves at Clinical Trial Steering Committees for Sanofi Genzyme and Takeda is Principal Investigator for UCB's CIDP01 trial and Sanofi's Mobilize and Vitalize trials. C.S. is supported by Deutsche Forschungsgemeinschaft (SFB1158, CRU5001 and RTG2026), unrelated to the content of this manuscript, and she is a Board Member of the Peripheral Nerve Society. She has served as a scientific advisor for Algiax, Grifols, Immunic, Kedrion, Sanofi, and Takeda, and has given educational talks for Alnylam, Argenx, CSL Behring, Grifols, Kedrion, and Takeda. P.W. is a named inventor on patents for antibody assays and has received royalties. He has received honoraria from Biogen Idec, Mereo Biopharma, Retrogenix, UBC, Euroimmun AG, UCB, F. Hoffmann La‐Roche and Alexion; travel grants from the Guthy‐Jackson Charitable Foundation; and research funding from Euroimmun AG. Work in the Autoimmune Neurology Diagnostic Laboratory is supported by the NHS Commissioning service for NMOSD. Hugh Willison, Susan Halstead, Victor Mgbachi, and Sophia Rohrbacher report no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

