Comparative transcriptomics and metabolomics provide insight into degeneration-related physiological mechanisms of Morchella importuna after long-term preservation
- PMID: 39887921
- PMCID: PMC11781861
- DOI: 10.1111/1751-7915.70045
Comparative transcriptomics and metabolomics provide insight into degeneration-related physiological mechanisms of Morchella importuna after long-term preservation
Abstract
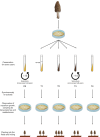
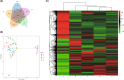
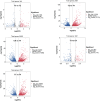
Ascomycetes fungi are often prone to degeneration. Agricultural production of the prized ascomycete mushroom Morchella importuna (black morel) typically suffers from reduced yield and malformed ascocarps owing to culture degeneration. This study compared M. importuna cultures subjected to five different long-term preservation treatments, using transcriptomics and metabolomics. Avoiding repeated subculturing in combination with nutrient-limited conditions was found to be the most beneficial method for maintaining the fruiting capability of morels. The expression of the gene sets involved in cysteine and methionine metabolism and nucleocytoplasmic transport was upregulated under nutrient-limited and nutrient-rich conditions, respectively. This increased expression was accompanied by differential accumulation of metabolites involved in nucleobase metabolism. Repeated subculturing triggered dissimilar changes in the functional modules under nutrient-rich and nutrient-limited conditions. A diverse set of cellular biochemical processes related to carbon metabolism were altered by repeated subculturing under nutrient-rich conditions, whereas glycerophospholipid and purine metabolism were key functions affected by repeated subculturing under nutrient-limited conditions. Altogether, metabolic alterations related to sulfur-containing amino-acid biosynthesis, DNA repair, and cellular structural maintenance contributed to improved preservation outcomes in terms of morel fruiting capability. Our findings contribute to a more detailed understanding of the molecular mechanisms related to subculturing and fruiting of ascomycete macrofungi after long-term preservation.
© 2025 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest associated with this work.
Figures




References
-
- Abdallah, R. , Rizk, I. , Hassan, A. & Elsheshetawy, H. (2021) A comparative study between different preservation methods on the viability of some yeast cultures. Arab Universities Journal of Agricultural Sciences, 29, 1–10.
-
- Aguilar, J.S. , Dulay, R.M.R. , Kalaw, S.P. & Reyes, R.G. (2024) Low‐cost preservation protocol for Lentinus mushrooms. Italian Journal of Mycology, 53, 99–109.
-
- Al‐Bedak, O.A. , Sayed, R.M. & Hassan, S.H.A. (2019) A new low‐cost method for long‐term preservation of filamentous fungi. Biocatalysis and Agricultural Biotechnology, 22, 101417.
-
- Ayala‐Zermeño, M.A. , Gallou, A. , Berlanga‐Padilla, A.M. , Andrade‐Michel, G.Y. , Rodríguez‐Rodríguez, J.C. , Arredondo‐Bernal, H.C. et al. (2017) Viability, purity, and genetic stability of entomopathogenic fungi species using different preservation methods. Fungal Biology, 121, 920–928. - PubMed
Publication types
MeSH terms
Grants and funding
- SCCXTD-2024-07/Sichuan Edible Mushroom Innovation Team of National Modern Agriculture Industry Technology System
- Sichuan Science and Technology Planning Project Key Research and Development Project (2021YFYZ0026)
- National Modern Agriculture Industry Technology System (CARS-20)
- Innovation 2035 Project of SAAS (YSCX2035-009)
- 1+9 Program of SAAS (1+9KJGG003)
- National Natural Science Foundation of China (NSFC31901119)
- National Scientific Observation and Experimental Station of Agro-Microorganisms, Xindu (NAES085AM05)
- Laboratory of Excellence ARBRE (ANR-11-LABX-0002-01)
- the Northwest Institute of Eco-Environment and Resources, and the Scientific and Technological Projects of Gansu Province (23YFFA0013)
LinkOut - more resources
Full Text Sources

