Identification of intraductal-to-invasive spatial transitions in prostate cancer: proposal for a new unifying model on intraductal carcinogenesis
- PMID: 39888049
- PMCID: PMC12045775
- DOI: 10.1111/his.15414
Identification of intraductal-to-invasive spatial transitions in prostate cancer: proposal for a new unifying model on intraductal carcinogenesis
Abstract
Aims: Intraductal carcinoma (IDC) is an independent pathological parameter for adverse prostate cancer (PCa) outcome. Although most IDC are believed to originate from retrograde spread of established PCa, rare IDC cases may represent precursor lesions. The actual transition areas between intraductal and invasive cancer, however, have not yet been identified. Our objective was to identify intraductal-invasive PCa transitions using 2- and 3-dimensional microscopy.
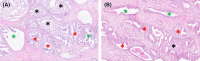
Methods and results: Seventy-five samples from 46 radical prostatectomies with PCa were immunohistochemically stained for basal cell keratins. In 35 samples, atypical glands that were indistinguishable from invasive adenocarcinoma (IAC) had focal 34BE12-positive basal cells. These IAC-like glands were present adjacent to IDC and prostatic intra-epithelial neoplasia (PIN) in 21 of 45 (46.7%) and 16 of 58 (27.6%) cases, respectively. Whole-mount confocal imaging of immunofluorescent Ker5/18 double-stained and cleared 1-mm-thick intact tissues revealed spatial continuity between IDC, IAC-like glands and IAC with a gradual loss of basal cells. In 24 of 35 (68.6%) samples more than one IAC-like focus (median 3.0) was present.
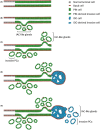
Conclusions: We identified areas of spatial transition between PIN, IDC and IAC, characterised by remnant basal cells in IAC-like glands. Based on the coexistence of IDC and PIN, the gradual loss of basal cells in IAC-like glands and IAC-like glands' multifocality, we propose a novel hypothesis on intraductal carcinogenesis, which we term 'repetitive invasion, precursor progression' (RIPP).
Keywords: IDC; PTEN; basal cell; intraductal; prostate cancer; three‐dimensional; transition.
© 2025 The Author(s). Histopathology published by John Wiley & Sons Ltd.
Conflict of interest statement
None of the authors declare any conflicts of interest.
Figures



References
-
- Moch H. WHO classification of tumours: urinary and male genital tumours. 5th ed. International Agency for Research on Cancer (IACR), 2022. Lyon, France.
-
- Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: histologic features and clinical significance. Mod. Pathol. 2006; 19; 1528–1535. - PubMed
-
- Kweldam CF, Kümmerlin IP, Nieboer D et al. Disease‐specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod. Pathol. 2016; 29; 630–636. - PubMed
-
- McKenney JK, Wei W, Hawley S et al. Histologic grading of prostatic adenocarcinoma can be further optimized: analysis of the relative prognostic strength of individual architectural patterns in 1275 patients from the canary retrospective cohort. Am. J. Surg. Pathol. 2016; 40; 1439–1456. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

