Discontinuation and Reinitiation of Dual-Labeled GLP-1 Receptor Agonists Among US Adults With Overweight or Obesity
- PMID: 39888616
- PMCID: PMC11786232
- DOI: 10.1001/jamanetworkopen.2024.57349
Discontinuation and Reinitiation of Dual-Labeled GLP-1 Receptor Agonists Among US Adults With Overweight or Obesity
Abstract
Importance: Adherence to glucagon-like peptide-1 receptor agonists (GLP-1 RAs) is important for their effectiveness. Discontinuation and reinitiation patterns are not well understood.
Objective: To describe rates of and factors associated with discontinuation and subsequent reinitiation of GLP-1 RAs among adults with overweight or obesity.
Design, setting, and participants: In this retrospective cohort study, 125 474 adults with overweight or obesity newly initiated treatment with a dual-labeled GLP-1 RA (liraglutide, semaglutide, or tirzepatide) between January 1, 2018, and December 31, 2023, with a baseline body mass index of 27 or more, an available weight measurement within 60 days before initiation, and regular care in the year before initiation were identified using electronic health record data from a collective of US health care systems. Patients were followed up for up to 2 years to assess discontinuation and for 2 additional years to assess reinitiation.
Exposure: Patients were stratified by presence of type 2 diabetes at baseline.
Main outcomes and measures: Proportions of patients discontinuing and reinitiating GLP-1 RA were estimated from Kaplan-Meier models. Associations of sociodemographic characteristics, health factors, weight changes, and gastrointestinal adverse events with discontinuation and reinitiation outcomes were modeled using time-varying Cox proportional hazards regression models. All analyses were conducted separately for patients with and patients without type 2 diabetes.
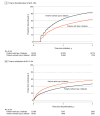
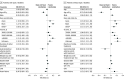
Results: In this cohort study of 125 474 adults (mean [SD] age, 54.4 [13.1] years; 82 063 women [65.4%]), 76 524 (61.0%) had type 2 diabetes. One-year discontinuation was significantly higher for patients without type 2 diabetes (64.8% [95% CI, 64.4%-65.2%]) compared with those with type 2 diabetes (46.5% [95% CI, 46.2%-46.9%]). Higher weight loss (1% reduction in weight from baseline was associated with a 3.1% [95% CI, 2.9%-3.2%] lower hazard of discontinuation for patients with type 2 diabetes and a 3.3% [95% CI, 3.2%-3.5%] lower hazard of discontinuation for patients without type 2 diabetes) and higher income (type 2 diabetes only; >$80 000: hazard ratio [HR], 0.72 [95% CI, 0.69-0.76]) were significantly associated with lower rates of discontinuation, while moderate or severe incident gastrointestinal adverse events were associated with a higher hazard of discontinuation (with type 2 diabetes: HR, 1.38 [95% CI, 1.31-1.45]; without type 2 diabetes: HR, 1.19 [95% CI, 1.12-1.27]). Of 41 792 patients who discontinued and had a discontinuation weight measurement available, 1-year reinitiation was lower for those without type 2 diabetes (36.3% [95% CI, 35.6%-37.0%]) compared with those with type 2 diabetes (47.3% [95% CI, 46.6%-48.0%]). Weight regain of 1% from discontinuation was significantly associated with increased hazards of reinitiation of 2.3% (95% CI, 1.9%-2.8%) for patients with type 2 diabetes and 2.8% (95% CI, 2.4%-3.2%) for patients without type 2 diabetes.
Conclusions and relevance: In this cohort study, most patients with overweight or obesity discontinued GLP-1 RA therapy within 1 year, but those without type 2 diabetes had higher discontinuation rates and lower reinitiation rates. Inequities in access and adherence to effective treatments have the potential to exacerbate disparities in obesity.
Conflict of interest statement
Figures

References
-
- Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960-1962 through 2017-2018. National Center for Health Statistics. 2020. Accessed August 24, 2021. https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm
-
- Davies M, Færch L, Jeppesen OK, et al. ; STEP 2 Study Group . Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi: 10.1016/S0140-6736(21)00213-0 - DOI - PubMed
-
- Garvey WT, Frias JP, Jastreboff AM, et al. ; SURMOUNT-2 investigators . Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402(10402):613-626. doi: 10.1016/S0140-6736(23)01200-X - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

