Optimal duration of ex vivo lung perfusion for heat stress-mediated therapeutic reconditioning of damaged rat donor lungs
- PMID: 39888846
- PMCID: PMC11831693
- DOI: 10.1093/ejcts/ezaf027
Optimal duration of ex vivo lung perfusion for heat stress-mediated therapeutic reconditioning of damaged rat donor lungs
Abstract
Objectives: Transient heat stress (HS) application during experimental ex vivo lung perfusion (EVLP) of warm ischaemic (WI) rat lungs produces a range of therapeutic benefits. Here, we explored whether different EVLP durations after HS application would influence its therapeutic effects.
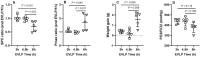
Methods: In protocol 1, WI rat lungs were exposed to HS (41.5°C, 60-90 min EVLP), and EVLP was maintained for 3, 4.5 or 6 h (n = 5/group), followed by physiological measurements (compliance, oedema, oxygenation capacity). In protocol 2, WI rat lungs treated with (HS groups) or without HS (control groups) were maintained for 3 or 4.5 h EVLP (n = 5/group), followed by physiological evaluation and measurements (lung tissue) of heat shock proteins (HSP70, HSP27, HS90, GRP78), endogenous proteins (surfactant protein-D, CC16, platelet endothelial cell adhesion molecule-1), anti-apoptotic (Bcl2, Bcl-xL) and pro-apoptotic proteins (Bcl2-associated X protein, CCAAT/enhancer binding-protein homologous protein), antioxidant enzymes (heme-oxygenase-1, nicotinamide di-phospho-nucleotide dehydrogenase quinone-1) and nitrotyrosine (oxidative stress biomarker).
Results: In protocol 1, physiological variables were stable after 3 and 4.5 h but deteriorated after 6 h. In protocol 2, at 3 h EVLP, HS-treated lungs differed from controls by higher expression of HSP70 and heme-oxygenase-1, and lower CC16 expression. In contrast, at 4.5 h EVLP, HS-treated lungs displayed improved physiology, higher levels of all HSPs, preserved or increased expression of surfactant protein-D, CC-16 and platelet endothelial cell adhesion molecule-1, increased antioxidant and anti-apoptotic proteins, and reduced pro-apoptotic proteins and nitrotyrosine.
Conclusions: The protective effects of HS application during EVLP of WI-damaged rat lungs strictly depend on the duration of post-HS recovery. An EVLP duration of 4.5 h appears to optimize the therapeutic potential of HS, while maintaining lungs in a stable physiological state.
Keywords: Ex vivo lung perfusion; Animal model; Heat shock response; Heat stress reconditioning; Warm ischaemia.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Figures

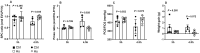
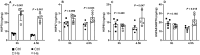



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

