Rapid expansion and specialization of the TAS2R bitter taste receptor family in amphibians
- PMID: 39888968
- PMCID: PMC11798467
- DOI: 10.1371/journal.pgen.1011533
Rapid expansion and specialization of the TAS2R bitter taste receptor family in amphibians
Abstract
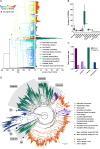
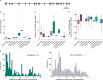
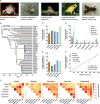
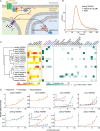
TAS2Rs are a family of G protein-coupled receptors that function as bitter taste receptors in vertebrates. Mammalian TAS2Rs have historically garnered the most attention, leading to our understanding of their roles in taste perception relevant to human physiology and behaviors. However, the evolution and functional implications of TAS2Rs in other vertebrate lineages remain less explored. Here, we identify 9,291 TAS2Rs from 661 vertebrate genomes. Large-scale phylogenomic analyses reveal that frogs and salamanders contain unusually high TAS2R gene content, in stark contrast to other vertebrate lineages. In most species, TAS2R genes are found in clusters; compared to other vertebrates, amphibians have additional clusters and more genes per cluster. We find that vertebrate TAS2Rs have few one-to-one orthologs between closely related species, although total TAS2R count is stable in most lineages. Interestingly, TAS2R count is proportional to the receptors expressed solely in extra-oral tissues. In vitro receptor activity assays uncover that many amphibian TAS2Rs function as tissue-specific chemosensors to detect ecologically important xenobiotics.
Copyright: © 2025 Higgins et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: J.-K.W. is a member of the Scientific Advisory Board and a shareholder of DoubleRainbow Biosciences, Galixir and Inari Agriculture, which develop biotechnologies related to natural products, drug discovery, and agriculture. The remaining authors declare no other competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

