Baculoviruses manipulate host lipid metabolism via adipokinetic hormone signaling to induce climbing behavior
- PMID: 39888969
- PMCID: PMC11819524
- DOI: 10.1371/journal.ppat.1012932
Baculoviruses manipulate host lipid metabolism via adipokinetic hormone signaling to induce climbing behavior
Abstract
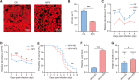
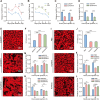
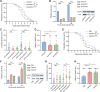
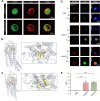
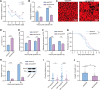
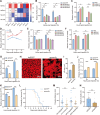
Baculoviruses can induce climbing behavior in caterpillar hosts, which provides an excellent model for studying parasite manipulation of host behavior. Herein, we found that Helicoverpa armigera single nucleopolyhedrovirus (HearNPV) promoted lipid metabolism of infected H. armigera larvae, and changes in lipid metabolism can affect climbing behavior. Therefore, understanding the molecular mechanisms between lipid metabolism and climbing behavior is particularly important. In this study, we found adipokinetic hormone 1 (HaAKH1), adipokinetic hormone 2 (HaAKH2) and their receptor HaAKHR were essential for promoting lipid metabolism and climbing behavior in response to HearNPV infection. Both molecular docking result and Ca2+ imaging showed that both HaAKH1 and HaAKH2 could interact with HaAKHR. Knockdown of HaAKH1, HaAKH2 and HaAKHR resulted in not only the accumulation of triacylglycerol (TAG), but also the reduction of the replication of HearNPV and the crawling ability of infected H. armigera larvae, resulting in a decrease in the final death height of the infected larvae. We further validated this conclusion by injecting active peptides of HaAKH1 and HaAKH2 to infected larvae. In addition, we investigated the downstream of HaAKH signaling and found that hormone-sensitive lipase (HaHSL) changed with changes in HaAKH signaling and HaHSL played the same role as HaAKH signaling. These findings not only revealed the mechanism by which parasites manipulated host lipid metabolism, but more significantly, explored the relationship between lipid metabolism and behavioral changes of hosts manipulated by parasites, broadening our understanding of the phenomenon of parasites manipulating host behavioral changes.
Copyright: © 2025 Zhu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- van Houte S, Ros VID, van Oers MM. Hyperactivity and tree-top disease induced by the baculovirus AcMNPV in Spodoptera exigua larvae are governed by independent mechanisms. Naturwissenschaften. 2014;101(4):347–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

